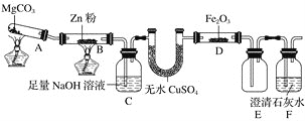
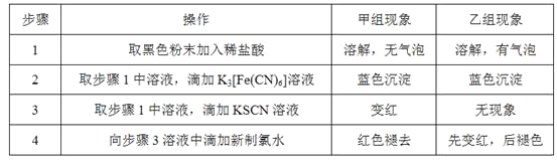
ƒøƒ⁄»ð
°æƒø°øªØ∫œŒÔM «…˙≤˙÷Œ¡∆∆§∑Ù≤°“©ŒÔµƒ÷–º‰Ã°£ µ—È “”…∑ºœ„◊ªØ∫œŒÔA÷∆±∏Mµƒ“ª÷÷∫œ≥…¬∑œþ»ÁÕº£∫
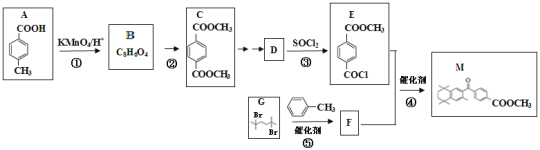
“—÷™£∫¢ŸRCOOH![]() RCOCl
RCOCl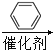
¢⁄![]()
![]()
![]() £®R±Ì æÃ˛ª˘£©
£®R±Ì æÃ˛ª˘£©
ªÿ¥œ¬¡–Œ £∫
£®1£©BµƒªØ—ß√˚≥∆Œ™___°£
£®2£©∑¥”¶¢⁄ ‘º¡∫ÕÃıº˛∑÷±Œ™___°£
£®3£©¢€µƒ∑¥”¶¿ý–Õ «___°£
£®4£©M÷–∫¨—ıπŸƒÐÕ≈µƒ√˚≥∆ «___°£
£®5£©∑¥”¶¢ðµƒªØ—ß∑Ω≥Ã ΩŒ™___°£
£®6£©–¥≥ˆÕ¨ ±¬˙◊„œ¬¡–Ãıº˛£¨”ÎDª•Œ™Õ¨∑÷“Ïππõƒ∑ºœ„◊ªØ∫œŒÔµƒΩ·ππºÚ Ω___£®»Œ–¥¡Ω÷÷£©°£
¢°.ƒÐ”ÎNaHCO3»Ð“∫∑¥”¶£ª
¢¢.ƒÐ∑¢…˙“¯æµ∑¥”¶£ª¢£.∫À¥≈π≤’Ò«‚∆◊÷–µƒ∑Â√ʪ˝÷Ʊ»Œ™1£∫2£∫2£∫2£∫1°£
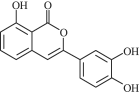
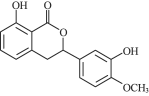
£®7£©≤Œ’’…œ ˆ∫œ≥…¬∑œþ∫Õ–≈œ¢£¨“‘±Ω∫Õ“ª‰Âº◊ÕÈŒ™‘≠¡œ£®ŒÞª˙ ‘º¡»Œ—°£©£¨…˺∆÷∆±∏![]() µƒ∫œ≥…¬∑œþ___°£
µƒ∫œ≥…¬∑œþ___°£
°æ¥∞∏°ø∂‘±Ω∂˛º◊À· CH3OH°¢≈®¡ÚÀ·£¨º”»» »°¥˙∑¥”¶ Ù ª˘°¢ı•ª˘£®ªÚı•º¸£© 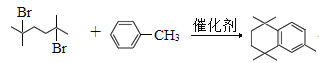 +2HBr
+2HBr ![]() ªÚ
ªÚ![]() ªÚ
ªÚ![]() ªÚ
ªÚ![]() ªÚ
ªÚ![]()
![]()
![]()
![]()
![]()
![]()
![]()
°æΩ‚Œˆ°ø
∏˘æð”–ª˙ŒÔ¡˜≥ÃÕ∆∂œ£¨A”ÎÀ·–‘∏þ√ÃÀ·ºÿ∑¢…˙—ıªØ∑¥”¶£¨…˙≥…B£¨º¥∂‘±Ω∂˛º◊À·£¨”κ◊¥º∑¢…˙ı•ªØ∑¥”¶…˙≥…C£ª∏˘æðƒÊÕ∆∑®£¨D”ÎSOCl2∑¥”¶…˙≥…E£¨º¥DŒ™CH3OOC-C6H4-COOH£¨G”κ◊±Ω∑¥”¶…˙≥…F£¨º¥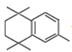 £¨◊Ó∫Û”ÎE∑¢…˙»°¥˙∑¥”¶…˙≥…M°£
£¨◊Ó∫Û”ÎE∑¢…˙»°¥˙∑¥”¶…˙≥…M°£
£®1£©A”ÎÀ·–‘∏þ√ÃÀ·ºÿ∑¥”¶…˙≥…B£¨ªØ—ß√˚≥∆Œ™ ∂‘±Ω∂˛º◊À·°£
£®2£©∑¥”¶¢⁄Œ™ı•ªØ∑¥”¶£¨B”ÎCH3OH∑¥”¶£¨Ãıº˛Œ™≈®¡ÚÀ·£¨º”»»°£
£®3£©∏˘æð∑÷◊” Ω≤Óø…÷™£¨¢€µƒ∑¥”¶¿ý–Õ «»°¥˙∑¥”¶°£
£®4£©”…MΩ·ππºÚ Ωø…÷™£¨M÷–∫¨—ıπŸƒÐÕ≈µƒ√˚≥∆ «Ù ª˘°¢ı•ª˘£®ªÚı•º¸£©°£
£®5£©∏˘æðÂ÷–‘≠¿Ìø…÷™£¨∑¥”¶¢ðµƒªØ—ß∑Ω≥Ã ΩŒ™ +2HBr°£
+2HBr°£
£®6£©¢°.ƒÐ”ÎNaHCO3»Ð“∫∑¥”¶£¨Àµ√˜”–ª˙ŒÔ∫¨”–Ù»ª˘£ª¢¢.ƒÐ∑¢…˙“¯æµ∑¥”¶£¨Àµ√˜”–ª˙ŒÔ∫¨”–»©ª˘ªÚ-OOCH£ª¢£.∫À¥≈π≤’Ò«‚∆◊÷–µƒ∑Â√ʪ˝÷Ʊ»Œ™1£∫2£∫2£∫2£∫1Àµ√˜»°¥˙ª˘Œ™∂‘Œª»°¥˙£¨º¥”ÎDª•Œ™Õ¨∑÷“Ïππõƒ∑ºœ„◊ªØ∫œŒÔµƒΩ·ππºÚ Ω![]() ªÚ
ªÚ![]() ªÚ
ªÚ![]() ªÚ
ªÚ![]() ªÚ
ªÚ![]() °£
°£
£®7£©∏˘æðƒÊÕ∆∑®£¨±Ω∫Õ“ª‰Âº◊ÕÈŒ™‘≠¡œ£¨÷∆±∏![]() µƒ∫œ≥…¬∑œþŒ™
µƒ∫œ≥…¬∑œþŒ™![]()
![]()
![]()
![]()

![]()
![]() °£
°£

°æƒø°ø𧓵»º…’√∫°¢ ؔյ»ªØ Ø»º¡œ Õ∑≈≥ˆ¥Û¡øµ™—ıªØŒÔ(NOx)°¢CO2°¢SO2µ»∆¯Ã£¨—œ÷ÿŒ€»æø’∆¯°£∂‘∑œ∆¯Ω¯––Õ—œı°¢Õ—ú∫ÕÕ—¡Ú¥¶¿Ìø… µœ÷¬Ã…´ª∑±£°¢∑œŒÔ¿˚”√°£
I. Õ—œı£∫
“—÷™£∫H2µƒ»º…’»»Œ™285.8kJ°§mol1
N2(g)+2O2(g)=2NO2(g) ¶§H=+133kJ°§mol1
H2O(g)=H2O(l) ¶§H=44kJ°§mol1
¥þªØº¡¥Ê‘⁄œ¬£¨H2ªπ‘≠NO2…˙≥…ÀÆ’Ù∆¯∫Õ∆‰À˚ŒÞ∂æŒÔ÷ µƒ»»ªØ—ß∑Ω≥Ã ΩŒ™£∫______°£
II. ՗ú£∫
(1)œÚ2L√б’»ð∆˜÷–º”»Î2mol CO2∫Õ6mol H2£¨‘⁄ µ±µƒ¥þªØº¡◊˜”√œ¬£¨∑¢…˙∑¥”¶£∫CO2(g)+3H2(g)![]() CH3OH(l)+H2O(l)
CH3OH(l)+H2O(l)
¢Ÿ ∏√∑¥”¶◊‘∑¢Ω¯––µƒÃıº˛ «_____(ÃÓ°∞µÕŒ¬°±°¢°∞∏þŒ¬°±ªÚ°∞»Œ“‚Œ¬∂»°±)
¢⁄ œ¬¡–– ˆƒÐÀµ√˜¥À∑¥”¶¥ÔµΩ∆Ω∫‚◊¥Ã¨µƒ «_____°£(ÃÓ◊÷ƒ∏)
a. ªÏ∫œ∆¯Ãµƒ∆Ωæ˘ Ω¡ø±£≥÷≤ª±‰ b. CO2∫ÕH2µƒÃª˝∑÷ ˝±£≥÷≤ª±‰ c. CO2∫ÕH2µƒ◊™ªØ¬ œýµ»
d. ªÏ∫œ∆¯Ãµƒ√Ð∂»±£≥÷≤ª±‰ e. 1 mol CO2…˙≥…µƒÕ¨ ±”–3 mol HHº¸∂œ¡—
(2) ∏ƒ±‰Œ¬∂»£¨ π∑¥”¶CO2(g)+3H2(g)![]() CH3OH(g)+H2O(g) ¶§H£º0÷–µƒÀ˘”–ŒÔ÷ ∂ºŒ™∆¯Ã¨°£∆ ºŒ¬∂»°¢Ãª˝œýÕ¨(T1°Ê°¢2L√б’»ð∆˜)°£∑¥”¶π˝≥Ã÷–≤ø∑÷ ˝æðº˚±Ì£∫
CH3OH(g)+H2O(g) ¶§H£º0÷–µƒÀ˘”–ŒÔ÷ ∂ºŒ™∆¯Ã¨°£∆ ºŒ¬∂»°¢Ãª˝œýÕ¨(T1°Ê°¢2L√б’»ð∆˜)°£∑¥”¶π˝≥Ã÷–≤ø∑÷ ˝æðº˚±Ì£∫
∑¥”¶ ±º‰ | CO2(mol) | H2(mol) | CH3OH(mol) | H2O(mol) | |
∑¥”¶I£∫∫„Œ¬∫„»ð | 0min | 2 | 6 | 0 | 0 |
10min | 4.5 | ||||
20min | 1 | ||||
30min | 1 | ||||
∑¥”¶II£∫毻»∫„»ð | 0min | 0 | 0 | 2 | 2 |
¢Ÿ¥ÔµΩ∆Ω∫‚ ±£¨∑¥”¶I°¢II∂‘±»£∫∆Ω∫‚≥£ ˝K(I)___K(II)(ÃÓ°∞£æ°±°∞£º°±ªÚ°∞=°±œ¬Õ¨)£ª∆Ω∫‚ ±CH3OHµƒ≈®∂»c(I)___c(II)°£
¢⁄∂‘∑¥”¶I£¨«∞10minƒ⁄µƒ∆Ωæ˘∑¥”¶ÀŸ¬ v(CH3OH)=_____°£‘⁄∆‰À˚Ãıº˛≤ª±‰µƒ«Èøˆœ¬£¨»Ù30 min ±÷ª∏ƒ±‰Œ¬∂»T2°Ê£¨¥À ±H2µƒŒÔ÷ µƒ¡øŒ™3.2 mol£¨‘ÚT1____T2(ÃÓ°∞£æ°±°¢°∞£º°±ªÚ°∞=°±)°£»Ù30 min ±÷ªœÚ»ð∆˜÷–‘Ÿ≥‰»Î1 mol CO2(g)∫Õ1 mol H2O(g)£¨‘Ú∆Ω∫‚_____“∆∂Ø(ÃÓ°∞’˝œÚ°±°¢°∞ƒÊœÚ°±ªÚ°∞≤ª°±)°£
°æƒø°ø±Ωº◊À· «“ª÷÷÷ÿ“™µƒªØπ§‘≠¡œ°£ µ—È “∫œ≥…±Ωº◊À·µƒ‘≠¿Ì°¢”–πÿ ˝æðº∞◊∞÷√ æ“‚Õº»Áœ¬:
√˚¥ | –‘◊¥ | »€µ„(°Ê) | ∑–µ„(°Ê) | √Ð∂»(g/mL) | »ÐΩ‚–‘ | |
ÀÆ | ““¥º | |||||
º◊±Ω | ŒÞ…´“∫Ó◊»º“◊ª”∑¢ | -95 | 110.6 | 0.8669 | ≤ª»Ð | ª•»Ð |
±Ωº◊À· | ∞◊…´∆¨◊¥ªÚ’Î◊¥æßà| 112.4(100°Ê◊Û”“…˝ª™) | 248 | 1.2659 | Œ¢»Ð | “◊»Ð |
±Ωº◊À·‘⁄ÀÆ÷–µƒ»ÐΩ‚∂»»Á±Ì£∫
Œ¬∂»/°Ê | 4 | 18 | 75 |
»ÐΩ‚∂»/g | 0.2 | 0.3 | 2.2 |
ƒ≥—ßœ∞–°◊È‘⁄ µ—È “÷∆±∏°¢∑÷¿Î°¢Ã·¥ø±Ωº◊À·£¨≤¢≤‚∂®À˘µ√—˘∆∑µƒ¥ø∂»£¨≤Ω÷Ë»Áœ¬:
“ª°¢÷∆±∏±Ωº◊À·
‘⁄b÷–º”»Î2.7mLº◊±Ω°¢100mL
∂˛°¢∑÷¿Î÷¥ø
‘⁄∑¥”¶ªÏ∫œŒÔ÷–º”»Î“ª∂®¡ø≤ðÀ·(H2C2O4)≥‰∑÷∑¥”¶£¨π˝¬À°¢œ¥µ”£¨Ω´¬À“∫∑≈‘⁄±˘ÀÆ‘°÷–¿‰»¥£¨»ª∫Û”√≈®—ŒÀ·À·ªØ£¨±Ωº◊À·»´≤øŒˆ≥ˆ∫Ûºı—ππ˝¬À£¨Ω´≥¡µÌŒÔ”√…Ÿ¡ø¿‰ÀÆœ¥µ”£¨º∑—π»•ÀÆ∑÷∫Û∑≈‘⁄∑–ÀÆ‘°…œ∏…‘Ô£¨µ√µΩ¥÷≤˙∆∑°£
»˝°¢≤‚∂®¥ø∂»
≥∆»°m g≤˙∆∑£¨≈‰≥…100mL““¥º»Ð“∫£¨“∆»°25.00mL»Ð“∫”⁄◊∂–Œ∆ø÷–£¨µŒº”2~3µŒ∑”Ù£¨»ª∫Û”√±Í◊º≈®∂»µƒKOH»Ð“∫µŒ∂®°£
«Îªÿ¥œ¬¡–Œ Â:
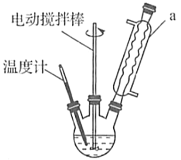
(1)◊∞÷√bµƒ√˚≥∆ «__________________£¨◊∞÷√aµƒ◊˜”√Œ™_____________________________°£
(2)∑÷¿Î÷¥øπ˝≥Ã÷–º”»Îµƒ≤ðÀ· «“ª÷÷∂˛‘™»ıÀ·£¨∑¥”¶π˝≥Ã÷–”–À· Ω—Œ°¢ŒÞ…´∆¯ÃÂ∫Õ∫⁄…´πÃÃÂ…˙≥…°£º”»Î≤ðÀ·µƒ◊˜”√ «_________________________£¨«Î”√¿Î◊”∑Ω≥Ã Ω±Ì æ∑¥”¶µƒ‘≠¿Ì______________________________°£
(3)≤˙∆∑ºı—ππ˝¬À ±”√¿‰ÀÆœ¥µ”µƒ‘≠“Ú «__________________________________________°£
(4)—°”√œ¬¡–__________≤Ÿ◊˜£¨ø…“‘Ω´¥÷≤˙∆∑Ω¯“ª≤Ω÷¥ø°£(—°ÃÓ◊÷ƒ∏)
A£Æ»Ð”⁄ÀÆ∫Ûπ˝¬À B£Æ»Ð”⁄““¥º∫Û’Ù¡Û C£Æ”√º◊±ΩðÕ»°∫Û∑÷“∫ D£Æ…˝ª™
(5)≤‚∂®¥ø∂»≤Ω÷Ë÷–£¨≈–∂œµŒ∂®÷’µ„µƒ±Í÷æ «________________________________________________°£»Ùm=1.200g£¨µŒ∂® ±”√»•0.1200mol°§ L£≠1±Í◊ºKOH»Ð“∫18.00mL£¨‘ÚÀ˘µ√≤˙∆∑÷–±Ωº◊À·µƒ÷ ¡ø∑÷ ˝Œ™__________(±£¡ÙÀƒŒª”––ß ˝◊÷)°£