��Ŀ����
����Ŀ��KMnO4��ʵ���Һ�ҵ�Ͼ�����ҪӦ�ã��乤ҵ�Ʊ��IJ��ֹ������£�
�����̿���Ҫ�ɷ�MnO2���������KOH�����ϣ�ͨ�������ֱ��գ����ɰ���ɫ����̬���ʡ�
����ȴ����������ϸ����ϡKOH��Һ��ȡ�����ˣ��ð���ɫ��Һ��
������ɫ��Һ��ͨ��CO2����Һ��Ϊ�Ϻ�ɫ��ͬʱ���ɺ�ɫ���塣
�������ˣ����Ϻ�ɫ��Һ����Ũ������ȴ�ᾧ�����ˣ�ϴ�ӣ������KMnO4���塣
���ϣ�K2MnO4Ϊ����ɫ���壬��ǿ������Һ���ȶ����ڽ����Ի�������Һ�������绯��Ӧ��Mn�Ļ��ϼۼ������ֽ��ͣ���
��1�����У��������̿��Ŀ����___________________��
��2�����У�����K2MnO4�Ļ�ѧ����ʽ��______________________________��
��3�����У���ȡʱ��ϡKOH��Һ��ԭ����_________________��
��4�����У�CO2��K2MnO4����Һ�з�Ӧ�Ļ�ѧ����ʽ��_____________________��
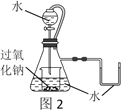
��5����K2MnO4��Һ���ö��Ե缫��Ĥ����⣬Ҳ���Ƶ�KMnO4��װ����ͼ��

��b����______�������������������D��_______________��
�ڽ�ϵ缫��Ӧʽ��������KMnO4��ԭ����_______________________________��
�۴�ͳ��Ĥ�����ʱ����Ԫ��������ƫ�ͣ���֮��ȣ��������ӽ���Ĥ���������Ԫ�ص������ʣ���ԭ����______��
��6���õζ����ⶨij������ز�Ʒ�Ĵ��ȣ��������£�
��֪��Na2C2O4+H2SO4=H2C2O4+Na2SO4��5H2C2O4+2MnO4-+6H+=2Mn2++10CO2��+8H2O��Ħ��������Na2C2O4134gmol-1��KMnO4158gmol-1��
������ȡag��Ʒ�����50mL��Һ��
������ȡbgNa2C2O4��������ƿ�У�������ˮʹ���ܽ⣬�ټ�����������ᡣ
��������ƿ����Һ���ȵ�75�桫80�棬���£��â���������Һ�ζ����յ㣬������ҺVmL�����ʲ����뷴Ӧ����
�ζ��յ������Ϊ_______________����Ʒ��KMnO4�����������ı���ʽΪ_____________��
���𰸡�����Ӧ��Ӵ�������ӿ췴Ӧ���� 2MnO2+4KOH+O2![]() 2K2MnO4+2H2O ������Һ��ǿ���ԣ���ֹK2MnO4�����绯��Ӧ 3K2MnO4+2CO2=2KMnO4+MnO2��+2K2CO3 �� ��Ũ��KOH��Һ a��MnO42--e-=MnO4-������K+ͨ�������ӽ���Ĥ����������������������KMnO4 �������ӽ���Ĥ�ɷ�ֹMnO4-��MnO42-����������ԭ ���������һ��KMnO4��Һ����ƿ�е�Һ������ɫ��Ϊdz��ɫ���Ұ�����ڲ��ָ�
2K2MnO4+2H2O ������Һ��ǿ���ԣ���ֹK2MnO4�����绯��Ӧ 3K2MnO4+2CO2=2KMnO4+MnO2��+2K2CO3 �� ��Ũ��KOH��Һ a��MnO42--e-=MnO4-������K+ͨ�������ӽ���Ĥ����������������������KMnO4 �������ӽ���Ĥ�ɷ�ֹMnO4-��MnO42-����������ԭ ���������һ��KMnO4��Һ����ƿ�е�Һ������ɫ��Ϊdz��ɫ���Ұ�����ڲ��ָ� ![]()
��������
���̢����̿���Ҫ�ɷ�MnO2�������Ŀ��������Ӧ��Ӵ�������ӿ췴Ӧ���ʣ��÷�Ӧ��Ҫ�����μӣ���Ӧ������״̬�½���Ŀ����ʹ�������ֽӴ�����������Ŀ��Ϣ��֪��MnO2��KOH�����ڻ������ͨ�����ʱ������Ӧ����K2MnO4������Ԫ���غ㻹Ӧ����ˮ�����ݵ��ӵ�ʧ��ԭ���غ������ƽ��
��K2MnO4Ϊ����ɫ���壬��ǿ������Һ���ȶ����ڽ����Ի�������Һ�������绯��Ӧ��Mn�Ļ��ϼۼ������ֽ��ͣ������Խ�������ϸ����ϡKOH��Һ��ȡ�����ˣ�
������ɫ��Һ��ͨ��CO2����Һ�����绯��Ӧ������KMnO4��MnO2��
�������ˣ����Ϻ�ɫ��Һ����Ũ������ȴ�ᾧ�����ˣ�ϴ�ӣ������KMnO4���塣
��5�����ص��K2MnO4��Һ�õ�KMnO4��֪��������ˮ�õ��ӷ�����ԭ��Ӧ�������������������ӣ����������������ʧ���ӷ�Ӧ������Ӧ���ɸ���������ӣ�
��6���ζ��յ㣬��Һ��ɫ�����仯���Ұ��������ɫ���䣻���ݷ�ӦNa2C2O4+H2SO4=H2C2O4+Na2SO4��5H2C2O4+2MnO4-+6H+=2Mn2++10CO2��+8H2O���ҳ���ϵʽ��Ȼ�����������ݼ��㡣
��1�����У��������̿��Ŀ����Ŀ��������Ӧ��Ӵ�������ӿ췴Ӧ���ʣ�
�𰸣� ����Ӧ��Ӵ�������ӿ췴Ӧ����
��2������Ŀ��Ϣ��֪��MnO2��KOH�����ڻ������ͨ�����ʱ������Ӧ����K2MnO4������Ԫ���غ㻹Ӧ����ˮ����Ӧ����Ԫ����+4������Ϊ+6�ۣ�����2�ۣ���Ԫ����0�۽���Ϊ-2�ۣ��ܹ�����4�ۣ����ϼ�������С������Ϊ4������MnO2ϵ��2��O2ϵ��Ϊ1��������Ԫ���غ�ȷ��K2MnO4ϵ��Ϊ2�����ݼ�Ԫ���غ�ȷ��KOHϵ��Ϊ4��������Ԫ���غ�ȷ��H2Oϵ��Ϊ2�����Է�Ӧ��ѧ����ʽΪ��2MnO2+4KOH+O2![]() 2K2MnO4+2H2O
2K2MnO4+2H2O
�𰸣�2MnO2+4KOH+O2![]() 2K2MnO4+2H2O
2K2MnO4+2H2O
��3���������Ϣ��֪��K2MnO4��ǿ������Һ���ȶ����ڽ����Ի�������Һ�������绯��Ӧ��Mn�Ļ��ϼۼ������ֽ��ͣ���
�𰸣�������Һ��ǿ���ԣ���ֹK2MnO4�����绯��Ӧ
��4����K2MnO4��Һ��ͨ��CO2����Һ��Ϊ�Ϻ�ɫKMnO4��ͬʱ���ɺ�ɫ����MnO2�����ݻ��ϼ�������Ⱥ������غ㶨��д������ʽ3K2MnO4+2CO2=2KMnO4+MnO2��+2K2CO3 ��
�𰸣�3K2MnO4+2CO2=2KMnO4+MnO2��+2K2CO3
��5����b���������������������缫��Ӧʽ��2H2O+2e-=H2��+2OH-��������ͨ�������ӽ���Ĥ��������������D�ǽ�Ũ��KOH��Һ��
�𰸣��� ��Ũ��KOH��Һ
��a����2MnO42--2e-=2MnO4-��������K+ͨ�������ӽ���Ĥ����������������������KMnO4��
�𰸣�a��MnO42--e-=MnO4-������K+ͨ�������ӽ���Ĥ����������������������KMnO4
�۴�ͳ��Ĥ�����ʱ����Ԫ��������ƫ�ͣ���֮��ȣ��������ӽ���Ĥ���������Ԫ�ص������ʣ���ԭ�����������ӽ���Ĥ�ɷ�ֹMnO4-��MnO42-��������������������ԭ��
�𰸣������ӽ���Ĥ�ɷ�ֹMnO4-��MnO42-����������ԭ
��6�����������һ��KMnO4��Һ����ƿ�е�Һ������ɫ��Ϊdz��ɫ���Ұ�����ڲ��ָ���
�����Ƶ�50ml���Ƶ���Ʒ��Һ�к��и������Ũ��Ϊcmol/L��
��5Na2C2O4��2KMnO4��
5mol 2mol
b/134 cV��10-3
�����c=![]() mol/L
mol/L
KMnO4�����������ı���ʽΪ��c��50��10-3��158/a =img src="http://thumb.zyjl.cn/questionBank/Upload/2019/06/26/08/9f783dd0/SYS201906260804278141572487_DA/SYS201906260804278141572487_DA.004.png" width="98" height="36" style="-aw-left-pos:0pt; -aw-rel-hpos:column; -aw-rel-vpos:paragraph; -aw-top-pos:0pt; -aw-wrap-type:inline" />��50��10-3��158/a= ![]() ��
��
�𰸣����������һ��KMnO4��Һ����ƿ�е�Һ������ɫ��Ϊdz��ɫ���Ұ�����ڲ��ָ� ![]()