��Ŀ����
17�����أ��Ҵ�����Դ���������ճ����������涼��ʮ�ֹ㷺��Ӧ�ã�����㣺��1���������Ҵ���9.2g�����Ƴ�ַ�Ӧ�����״������������������4.48L��
��2����һ�������Ҵ���O2���ܱ�������ȼ�պ�IJ���ΪCO2��CO��H2O��g�����������ξ���Ũ����ͼ�ʯ��ʹ�䱻������գ�Ũ��������10.8g����ʯ������13.2g�������������ʵ�����0.55mol��
��3��д�����Ա��Ҵ���һ��-CH2-ԭ��ͼ��ͬϵ��Ľṹ��ʽ��
���� ��1������n=$\frac{m}{M}$=$\frac{V}{{V}_{m}}$����ѧ����ʽ�б�����ϵ2Na��H2�����㣻
��2��Ũ��������10.8gΪ����ˮ������������C2H5OH��3H2O�����Ҵ������ʵ�������ʯ������13.2gΪ������̼������������̼ԭ���غ����CO�����ʵ�����Ȼ�������ԭ���غ�������������ʵ�����
��3�����Ҵ���һ��-CH2-ԭ���ŵ�ͬϵ��Ϊ������
��� �⣺��1�������⣺
2Na��H2��
46g 22.4L
9.2g V��H2��
V��H2��=$\frac{22.4L��9.2g}{46g}$=4.48L��
�ʴ�Ϊ��4.48��
��2��������ԭ���غ㣬�ɵã�
C2H5OH��3H2O
1mol 54g
n��C2H5OH�� 10.8g
n��C2H5OH��=$\frac{1mol��10.8g}{54g}$=0.2mol��
��ʯ�����ص�����Ϊ����CO2����������n��CO2��=$\frac{13.2g}{44g/mol}$=0.3mol��
����̼ԭ���غ㣺n��CO��=2��0.2mol-0.3mol=0.1mol��
������ԭ���غ㣺n��O2��=$\frac{1}{2}$��0.6mol+0.1mol+0.3mol��2-0.2mol��=0.55mol��
�ʴ�Ϊ��0.55��
��3�����Ҵ���һ��-CH2-ԭ���ŵ�ͬϵ��Ϊ��������ṹ��ʽΪCH3CH2CH2OH��CH3CHOHCH3��
�𣺱��Ҵ���һ��-CH2-ԭ���ŵ�ͬϵ��Ľṹ��ʽΪCH3CH2CH2OH��CH3CHOHCH3��
���� ���⿼���˻�ѧ����ʽ�ļ��㡢�ṹ��ʽ����д�������������ʵ���Ϊ���ĵļ��㹫ʽ��ԭ���غ��Ӧ�ã���Ŀ�ѶȲ���

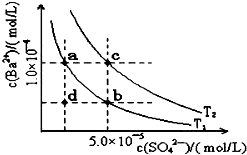 ��֪BaSO4��s��?Ba2+��aq��+SO4 2-��aq����25��ʱKsp=1.07��10-10����BaSO4�����¶����߶�������ͼ��ʾ����T1��T2��ͬ�¶�������BaSO4��ˮ�еij����ܽ�ƽ�����ߣ�������˵������ȷ���ǣ�������
��֪BaSO4��s��?Ba2+��aq��+SO4 2-��aq����25��ʱKsp=1.07��10-10����BaSO4�����¶����߶�������ͼ��ʾ����T1��T2��ͬ�¶�������BaSO4��ˮ�еij����ܽ�ƽ�����ߣ�������˵������ȷ���ǣ�������| A�� | �¶�ΪT1ʱ����T1�����Ϸ���������һ��ʱ������BaSO4�������� | |
| B�� | �����ܼ�����ʹ��Һ��d���ΪT1������a��b֮���ijһ�� | |
| C�� | ���¿�ʹ��Һ��b���Ϊd�� | |
| D�� | T2��25�� |
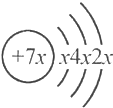 ��b��c�γɵĻ�����Ļ�ѧʽΪb3c����b��c��������ͬ�ĵ��Ӳ�ṹ�����бȽ��У���ȷ���ǣ�������
��b��c�γɵĻ�����Ļ�ѧʽΪb3c����b��c��������ͬ�ĵ��Ӳ�ṹ�����бȽ��У���ȷ���ǣ�������| A�� | ԭ��������a��b��c | B�� | �⻯����ȶ��ԣ�a��c��d | ||
| C�� | ԭ�Ӱ뾶��d��a��c | D�� | ��ۺ���������ԣ�d��c��a |
 ij�¶�ʱ����һ���ݻ�Ϊ2L���ܱ������У�X��Y��Z������������ʵ�����ʱ��ı仯������ͼ��ʾ������ͼ�����ݣ������й�˵��������ǣ�������
ij�¶�ʱ����һ���ݻ�Ϊ2L���ܱ������У�X��Y��Z������������ʵ�����ʱ��ı仯������ͼ��ʾ������ͼ�����ݣ������й�˵��������ǣ�������| A�� | ��Ӧ��ʼ��2min��Z�ķ�Ӧ����Ϊ0.10mol•L-1•min-1 | |
| B�� | �÷�Ӧ�Ļ�ѧ����ʽΪ3X+Y?2Z | |
| C�� | 2minʱ����Ӧ������ȣ�����ѧ��Ӧ���ڽ��� | |
| D�� | ��Ӧ�ﵽƽ��ʱ��ѹǿ�ǿ�ʼʱ��0.9�� |
| A�� | ǿ������Һ�У�K+��Al3+��Cl-��SO42- | |
| B�� | ����0.1 mol/L Fe3+����Һ�У�K+��Mg2+��I-��Cl- | |
| C�� | ����0.1 mol/L Ca2+����Һ�У�Na+��K+��SO42-��Cl- | |
| D�� | �����£�pH=1����Һ�У�Na+��Fe3+��NO-3��SO42- |
| A�� | ��Һ������NaOH | B�� | ���ʿ�����Cu2��OH��2CO3 | ||
| C�� | ��Һ��һ��������H+���� | D�� | ��Һ�ʼ��� |
| A�� | ����̿��C60�����ɰ����̼��ͬ�������� | |
| B�� | Fe2O3��Na2O2��K2O���Ǽ��������� | |
| C�� | ���ۡ������ʡ���֬�������л��߷��ӻ����� | |
| D�� | ����Ȼ�李������������Ҷ�����ǿ����� |
 �����ѣ�CH3OCH4����һ����Ҫ�����ȼ�ϣ���ҵ������ˮú���ϳɶ����ѵ�������Ӧ���£�
�����ѣ�CH3OCH4����һ����Ҫ�����ȼ�ϣ���ҵ������ˮú���ϳɶ����ѵ�������Ӧ���£�