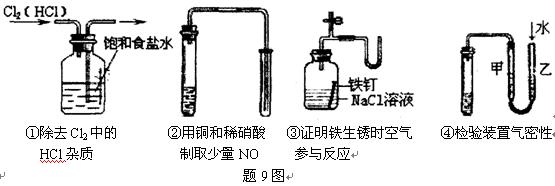
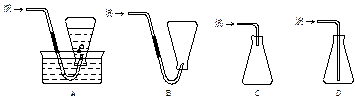��Ŀ����
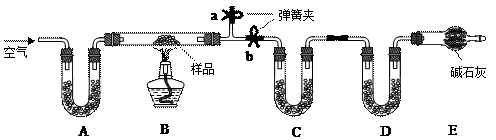
��13�֣��йش����Ĵ�������������Դӡ��Ҵ�������ʵ�顱�õ�һЩ��ʶ��ij��ʦ�������ͼװ�ã��г�װ��һ����ʡ�ԣ�����ʵ�����Ϊ���Ȱ�ͼ��װ�ã��ȹرջ���a��b��c����ͭ˿���м䲿�ּ���Ƭ�̣�Ȼ�����a��b��c��ͨ�����ƻ���b��c�����н��ࣨ��Ъ�ԣ�ͨ�����壬������M���۲쵽���Ե�ʵ�������Իش��������⣺
������C����ˮ�����ã�����������������������������������������������������������
�������ʹ������ķ�Ӧ�Ļ�ѧ����ʽΪ������������������������������������
�������ӣ��пɹ۲쵽����������������������������������������������������
���п���ʶ����ʵ������д�������������μӡ����μӡ�����ѧ��Ӧ����������ʶ����������Ҫһ����
��4��ʵ��һ��ʱ�����������ƾ��ƣ���Ӧ ����ܡ����ܡ����������У���ԭ��������������������������������������������������������������������������

������C����ˮ�����ã�����������������������������������������������������������
�������ʹ������ķ�Ӧ�Ļ�ѧ����ʽΪ������������������������������������
�������ӣ��пɹ۲쵽����������������������������������������������������
���п���ʶ����ʵ������д�������������μӡ����μӡ�����ѧ��Ӧ����������ʶ����������Ҫһ����
��4��ʵ��һ��ʱ�����������ƾ��ƣ���Ӧ ����ܡ����ܡ����������У���ԭ��������������������������������������������������������������������������
������C����ˮʹ�Ҵ������õ��Ҵ�����
������ ���������ż�Ъ�Ե�ͨ�����������Կ������Ȳ��ֵ�����ͭ������ֱ�ڡ��������μӡ��¶ȣ�4���ܣ������Ҵ��������Ƿ��ȷ�Ӧ���������ƾ��ƺ��ڷ�Ӧ�ų������������£�����ʹ��Ӧ�������С�
���������ż�Ъ�Ե�ͨ�����������Կ������Ȳ��ֵ�����ͭ������ֱ�ڡ��������μӡ��¶ȣ�4���ܣ������Ҵ��������Ƿ��ȷ�Ӧ���������ƾ��ƺ��ڷ�Ӧ�ų������������£�����ʹ��Ӧ�������С�
������
 ���������ż�Ъ�Ե�ͨ�����������Կ������Ȳ��ֵ�����ͭ������ֱ�ڡ��������μӡ��¶ȣ�4���ܣ������Ҵ��������Ƿ��ȷ�Ӧ���������ƾ��ƺ��ڷ�Ӧ�ų������������£�����ʹ��Ӧ�������С�
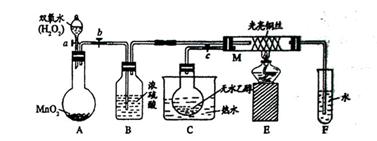
���������ż�Ъ�Ե�ͨ�����������Կ������Ȳ��ֵ�����ͭ������ֱ�ڡ��������μӡ��¶ȣ�4���ܣ������Ҵ��������Ƿ��ȷ�Ӧ���������ƾ��ƺ��ڷ�Ӧ�ų������������£�����ʹ��Ӧ�������С������Կα�ʵ��Ϊ����������ѧ����ʵ����������������̲ģ������ڽ̲ģ�ʵ���Ŀ����Ϊ����֤�Ҵ��Ĵ���������������P���������ʣ��õ�ҩƷΪ�Ҵ�������������ͭ��װ��A����ȡ������װ�ã���Ӧ�ķ���ʽΪ��2H2O2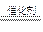 2H2O��O2����Ϊ�˵õ���������������ɵ�����ͨ��B�С���Cװ���ж���ˮ�Ҵ�����ˮԡ���ȣ��õ��Ҵ���������װ��M�У�������ͭ�������½��д�������Ӧ����Ӧ�ķ���ʽΪ��
2H2O��O2����Ϊ�˵õ���������������ɵ�����ͨ��B�С���Cװ���ж���ˮ�Ҵ�����ˮԡ���ȣ��õ��Ҵ���������װ��M�У�������ͭ�������½��д�������Ӧ����Ӧ�ķ���ʽΪ��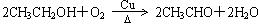 �����ż�Ъ�Ե�ͨ�����������Կ������Ȳ��ֵ�����ͭ������ֱ�ڡ���������δ���Ȳ��ֵ�����ͭ���䣬˵����ʵ������У������˻�ѧ��Ӧ��������Ҫһ�����¶Ȳ��������á��������Ҵ��������Ƿ��ȷ�Ӧ���������ƾ��ƺ��ڷ�Ӧ�ų������������£�����ʹ��Ӧ�������С�
�����ż�Ъ�Ե�ͨ�����������Կ������Ȳ��ֵ�����ͭ������ֱ�ڡ���������δ���Ȳ��ֵ�����ͭ���䣬˵����ʵ������У������˻�ѧ��Ӧ��������Ҫһ�����¶Ȳ��������á��������Ҵ��������Ƿ��ȷ�Ӧ���������ƾ��ƺ��ڷ�Ӧ�ų������������£�����ʹ��Ӧ�������С�
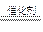 2H2O��O2����Ϊ�˵õ���������������ɵ�����ͨ��B�С���Cװ���ж���ˮ�Ҵ�����ˮԡ���ȣ��õ��Ҵ���������װ��M�У�������ͭ�������½��д�������Ӧ����Ӧ�ķ���ʽΪ��
2H2O��O2����Ϊ�˵õ���������������ɵ�����ͨ��B�С���Cװ���ж���ˮ�Ҵ�����ˮԡ���ȣ��õ��Ҵ���������װ��M�У�������ͭ�������½��д�������Ӧ����Ӧ�ķ���ʽΪ��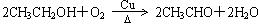 �����ż�Ъ�Ե�ͨ�����������Կ������Ȳ��ֵ�����ͭ������ֱ�ڡ���������δ���Ȳ��ֵ�����ͭ���䣬˵����ʵ������У������˻�ѧ��Ӧ��������Ҫһ�����¶Ȳ��������á��������Ҵ��������Ƿ��ȷ�Ӧ���������ƾ��ƺ��ڷ�Ӧ�ų������������£�����ʹ��Ӧ�������С�
�����ż�Ъ�Ե�ͨ�����������Կ������Ȳ��ֵ�����ͭ������ֱ�ڡ���������δ���Ȳ��ֵ�����ͭ���䣬˵����ʵ������У������˻�ѧ��Ӧ��������Ҫһ�����¶Ȳ��������á��������Ҵ��������Ƿ��ȷ�Ӧ���������ƾ��ƺ��ڷ�Ӧ�ų������������£�����ʹ��Ӧ�������С�
��ϰ��ϵ�д�
�����Ŀ