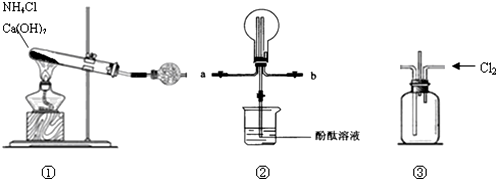
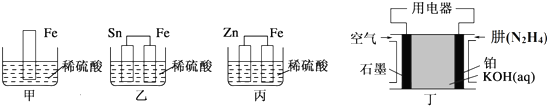
��Ŀ����
6��A��һ�־��ô��ᵯ����Ҫ�ɷ֣�H���Ƶø߾���PLA��������A ����Է�������Ϊ161��������C��H�⣬��������һ��±ԭ�ӣ�������ֻ����һ������������A��H ��ת����ϵ����ͼ��ʾ����������������Cu��OH��2����Һ��1mol C ��Ӧ������1mol Cu2O ��1mol D��B1��B2 ��Ϊͬ���칹�壬B1 ��Ħ������80g/mol��G1��G2 ��Ϊͬ���칹�壬���ߵĺ˴Ź�������ֻ�����������G1����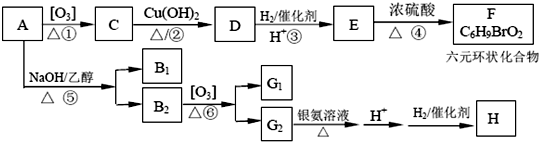
��֪��
��
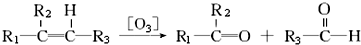
��һ��̼ԭ������������̼̼˫���Ľṹ��-C=C=C-�����ȶ���
������������⣺
��1��A �Ľṹ��ʽ��
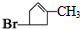 ��B1 �ķ���ʽΪC6H8��
��B1 �ķ���ʽΪC6H8����2����Ӧ�ܵĻ�ѧ����ʽ��HOOCCH2CHBrCH2CH��OH��CH3

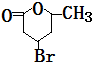 +H2O��
+H2O����3��д��H��һ�����������ɸ߾���ķ�Ӧ����ʽ
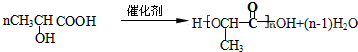 ��
����4����������������E ��ͬ���칹�干��2�֣�
�ٺ����������� ������NaHCO3 ��Ӧ�� ��-OH��-Br������ͬһ��̼ԭ���ϣ�
��5�����ʵ����C �������еķ���������ȡ����C��������������ˮ��Һ����ˮ�⣬Ȼ��������ữ���ټ�����������Һ������������ɫ��������˵��������ԭ�ӣ�
���� A��������F����6��Cԭ�ӡ�����1��Brԭ�ӣ���A�к���6��Cԭ�ӡ�1��Brԭ�ӣ�����ΪHԭ�ӣ�Hԭ�Ӹ���Ϊ161-80-6��12=9��A�ķ���ʽΪC6H9Br�������������IJ���ֻ��һ�֣���AӦ�ǻ�״���������ӽṹ��ֻ��һ��-CH3����AΪ��������Ԫ��״�����A�ij�������IJ���C��Cu��OH��2��Ӧ�ı�����ϵ֪C������ֻ��һ��ȩ������A�м��������е�һ��������̼ԭ���ϣ��ٽ����Ŀ���ṩ�Ľṹ��Ϣ֪��A�ṹΪ 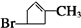 ������������Ϣ��֪A��������������C��CΪ
������������Ϣ��֪A��������������C��CΪ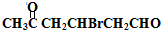 ��C����ΪD���������������ӳɷ�Ӧ�����������µõ�E����DΪ
��C����ΪD���������������ӳɷ�Ӧ�����������µõ�E����DΪ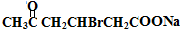 ��EΪHOOCCH2CHBrCH2CH��OH��CH3��E����������Ӧ����F����FΪ
��EΪHOOCCH2CHBrCH2CH��OH��CH3��E����������Ӧ����F����FΪ ��B1��A����ȥ��������ʽΪC6H8��B2��������õ�G1��G2��Ϊͬ���칹�壬���ߵĺ˴Ź�������ֻ�����������G1������B2Ϊ
��B1��A����ȥ��������ʽΪC6H8��B2��������õ�G1��G2��Ϊͬ���칹�壬���ߵĺ˴Ź�������ֻ�����������G1������B2Ϊ ��G1ΪOHC-CH2-CHO��G2ΪOHCCOCH3����ת����ϵ��֪HΪHOOCCH��OH��CH3���Դ˽��
��G1ΪOHC-CH2-CHO��G2ΪOHCCOCH3����ת����ϵ��֪HΪHOOCCH��OH��CH3���Դ˽��
��� �⣺A��������F����6��Cԭ�ӡ�����1��Brԭ�ӣ���A�к���6��Cԭ�ӡ�1��Brԭ�ӣ�����ΪHԭ�ӣ�Hԭ�Ӹ���Ϊ161-80-6��12=9��A�ķ���ʽΪC6H9Br�������������IJ���ֻ��һ�֣���AӦ�ǻ�״���������ӽṹ��ֻ��һ��-CH3����AΪ��������Ԫ��״�����A�ij�������IJ���C��Cu��OH��2��Ӧ�ı�����ϵ֪C������ֻ��һ��ȩ������A�м��������е�һ��������̼ԭ���ϣ��ٽ����Ŀ���ṩ�Ľṹ��Ϣ֪��A�ṹΪ  ������������Ϣ��֪A��������������C��CΪ
������������Ϣ��֪A��������������C��CΪ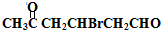 ��C����ΪD���������������ӳɷ�Ӧ�����������µõ�E����DΪ
��C����ΪD���������������ӳɷ�Ӧ�����������µõ�E����DΪ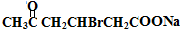 ��EΪHOOCCH2CHBrCH2CH��OH��CH3��E����������Ӧ����F����FΪ
��EΪHOOCCH2CHBrCH2CH��OH��CH3��E����������Ӧ����F����FΪ ��B1��A����ȥ��������ʽΪC6H8��B2��������õ�G1��G2��Ϊͬ���칹�壬���ߵĺ˴Ź�������ֻ�����������G1������B2Ϊ
��B1��A����ȥ��������ʽΪC6H8��B2��������õ�G1��G2��Ϊͬ���칹�壬���ߵĺ˴Ź�������ֻ�����������G1������B2Ϊ ��G1ΪOHC-CH2-CHO��G2ΪOHCCOCH3����ת����ϵ��֪HΪHOOCCH��OH��CH3��
��G1ΪOHC-CH2-CHO��G2ΪOHCCOCH3����ת����ϵ��֪HΪHOOCCH��OH��CH3��
��1�������Ϸ�����֪AΪ ��B1ΪC6H8���ʴ�Ϊ��
��B1ΪC6H8���ʴ�Ϊ�� ��C6H8��
��C6H8��
��2����Ӧ�ܵĻ�ѧ����ʽ��HOOCCH2CHBrCH2CH��OH��CH3
 +H2O��
+H2O��
�ʴ�Ϊ��HOOCCH2CHBrCH2CH��OH��CH3
 +H2O��
+H2O��
��3��H��һ�����������ɸ߾���ķ�Ӧ����ʽΪ�� ��
��
�ʴ�Ϊ�� ��
��
��4��E��ͬ���칹������������ٺ�����������������NaHCO3 ��Ӧ������-COOH����-OH��-Br������ͬһ��̼ԭ���ϣ������������E��ͬ���칹��Ϊ��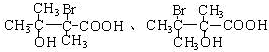 ��
��
�ʴ�Ϊ��2��
��5��CΪ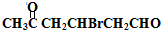 �����ʵ��������������еķ�����������ԭ�ӷ���Ϊ��ȡ����C��������������ˮ��Һ����ˮ�⣬Ȼ��������ữ���ټ�����������Һ������������ɫ��������˵��������ԭ�ӣ�
�����ʵ��������������еķ�����������ԭ�ӷ���Ϊ��ȡ����C��������������ˮ��Һ����ˮ�⣬Ȼ��������ữ���ټ�����������Һ������������ɫ��������˵��������ԭ�ӣ�
�ʴ�Ϊ��ȡ����C��������������ˮ��Һ����ˮ�⣬Ȼ��������ữ���ټ�����������Һ������������ɫ��������˵��������ԭ�ӣ�
���� ���⿼���л�����ƶϺͺϳɣ���Ŀ��Ϊ�ۺϣ��ѶȽϴ�����ʱע������ú������Ϣ���������������ϵķ����ƶϣ���ѧ�����������нϸߵ�Ҫ��ȷ��A�Ľṹ�ǹؼ���ע��ͬ���칹����Ŀ���жϷ�����

 ��ʦ����ɳ���ʱͬ��ѧ����ϵ�д�
��ʦ����ɳ���ʱͬ��ѧ����ϵ�д�| NaCl | MgCl2 | AlCl3 | SiCl4 | ����B | |
| �۵�/�� | 810 | 710 | 190 | -68 | 2 300 |
| �е�/�� | 1 465 | 1418 | 182.7 | 57 | 2 500 |
| A�� | SiCl4�Ƿ��Ӿ��� | B�� | ����B������ԭ�Ӿ��� | ||
| C�� | AlCl3���������� | D�� | KCl���۵����810�� |
| ���� | �����Լ� | ���ӷ���ʽ |
| NH4Cl��AlCl3����Һ | NH3•H2O | Al3++3NH3•H2O=Al��OH��3��+3NH4+ |
| NaHCO3��Na2CO3����Һ | CO2 | CO32-+CO2+H2O=2HCO3- |
| Fe �ۣ�Al�ۣ� | NaOH | 2Al+2OH-+2H2O=2AlO2-+3H2�� |
| FeCl3��FeCl2����Һ | Cl2 | 2Fe2++Cl2=2Fe3++2Cl- |
| A�� | ���ȳʺ�ɫ����Һ��NH4+��Ba2+��AlO2-��Cl- | |
| B�� | ��NaOH�����������ų����г������ɵ���Һ��Ca2+��HCO3-��NH4+��CH3COO- | |
| C�� | ������Ӧ����������������Һ��NH4+��Na+��CO32-��NO3- | |
| D�� | ����������ɫ����Һ��I-��K+��SO42-��Mg2+ |
| A�� | SO2��NO��CO���̳� | B�� | CO2��Cl2��N2������ | C�� | HCl��SO2��N2���۳� | D�� | CO2��NO��NH3���� |