题目内容
【题目】工业上用含锰废料(主要成分MnO2,含有少量Fe2O3、Al2O3、CuO、CaO等)与烟气脱硫进行联合处理并制备MnSO4的流程如下:

已知:25℃时,部分氢氧化物的溶度积常数(Ksp)如下表所示。
氢氧化物 | Al(OH)3 | Fe(OH)3 | Cu(OH)2 | Mn(OH)2 |
Ksp | 1.0×10-33 | 4.0×10-38 | 2.0×10-20 | 4.0×10-14 |
请回答:
(1)沉淀1的化学式为__________________。
(2)(NH4)2S的电子式为________________;“净化”时,加入(NH4)2S的作用为___________________。
(3)“酸化、还原”中,发生的所有氧化还原反应的离子方程式为__________________。
(4)已知:滤液3中除MnSO4外,还含有少量(NH4)2SO4。(NH4)2SO4、MnSO4的溶解度曲线如下图所示。
据此判断,操作“I”应为蒸发浓缩、____________、洗涤、干燥。

(5)工业上可用电解酸性MnSO4溶液的方法制备MnO2,其阳极反应式为________________。
(6)25.35 g MnSO4·H2O样品受热分解过程的热重曲线(样品质量随温度变化的曲线)如下图所示。
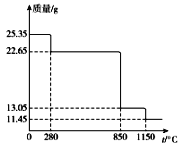
①300℃时,所得固体的化学式为______________________。
②1150℃时,反应的化学方程式为___________________。
【答案】 CaSO4  使Cu2+转化为CuS沉淀(或除去Cu2+) MnO2+SO2=Mn2++SO42- 、 Fe2O3+SO2+2H+=2Fe2++SO42-+H2O 趁热过滤 Mn2++2H2O-2e-=MnO2+4H+ MnSO4 3MnO2
使Cu2+转化为CuS沉淀(或除去Cu2+) MnO2+SO2=Mn2++SO42- 、 Fe2O3+SO2+2H+=2Fe2++SO42-+H2O 趁热过滤 Mn2++2H2O-2e-=MnO2+4H+ MnSO4 3MnO2 ![]() Mn3O4+O2↑
Mn3O4+O2↑
【解析】(1)硫酸钙微溶,因此沉淀1的化学式为CaSO4。(2)(NH4)2S是离子化合物,电子式为 ;滤液1中加入双氧水氧化亚铁离子为铁离子,根据氢氧化物的溶度积常数(Ksp) 可知pH=5时铝离子、铁离子沉淀完全,滤液2中还含有铜离子,因此“净化”时,加入(NH4)2S的作用为使Cu2+转化为CuS沉淀。(3)根据以上分析可知“酸化、还原”中,发生的所有氧化还原反应的离子方程式为MnO2+SO2=Mn2++SO42-、Fe2O3+SO2+2H+=2Fe2++SO42-+H2O。(4)硫酸锰的溶解度随温度升高达到最大值后继续升高温度溶解度降低,而硫酸铵的溶解度一直随温度升高而增大,所以操作“I”应为蒸发浓缩、趁热过滤、洗涤、干燥。(5)工业上可用电解酸性MnSO4溶液的方法制备MnO2,说明锰离子在阳极失去电子,则其阳极反应式为Mn2++2H2O-2e-=MnO2+4H+。(6)①25.35 g MnSO4·H2O的物质的量是25.35g÷169g/mol=0.15mol。其中结晶水的质量=0.15mol×18g/mol=2.7g,由于25.35g-2.7g=22.65g,所以300℃时,所得固体的化学式为MnSO4。②850℃时质量又减少22.65g-13.05g=9.6g,由于0.15molSO2恰好是9.6g,所以此时得到的物质是MnO2;1150℃时,固体质量又减少13.05g-11.45g=1.6g,即减少氧原子的质量是1.6g,物质的量是0.1mol,剩余氧原子的物质的量是0.15mol×2-0.1mol=0.2mol,即Mn和O的个数之比是0.15:0.2=3:4,所以得到的物质是Mn3O4,因此反应的化学方程式为3MnO2
;滤液1中加入双氧水氧化亚铁离子为铁离子,根据氢氧化物的溶度积常数(Ksp) 可知pH=5时铝离子、铁离子沉淀完全,滤液2中还含有铜离子,因此“净化”时,加入(NH4)2S的作用为使Cu2+转化为CuS沉淀。(3)根据以上分析可知“酸化、还原”中,发生的所有氧化还原反应的离子方程式为MnO2+SO2=Mn2++SO42-、Fe2O3+SO2+2H+=2Fe2++SO42-+H2O。(4)硫酸锰的溶解度随温度升高达到最大值后继续升高温度溶解度降低,而硫酸铵的溶解度一直随温度升高而增大,所以操作“I”应为蒸发浓缩、趁热过滤、洗涤、干燥。(5)工业上可用电解酸性MnSO4溶液的方法制备MnO2,说明锰离子在阳极失去电子,则其阳极反应式为Mn2++2H2O-2e-=MnO2+4H+。(6)①25.35 g MnSO4·H2O的物质的量是25.35g÷169g/mol=0.15mol。其中结晶水的质量=0.15mol×18g/mol=2.7g,由于25.35g-2.7g=22.65g,所以300℃时,所得固体的化学式为MnSO4。②850℃时质量又减少22.65g-13.05g=9.6g,由于0.15molSO2恰好是9.6g,所以此时得到的物质是MnO2;1150℃时,固体质量又减少13.05g-11.45g=1.6g,即减少氧原子的质量是1.6g,物质的量是0.1mol,剩余氧原子的物质的量是0.15mol×2-0.1mol=0.2mol,即Mn和O的个数之比是0.15:0.2=3:4,所以得到的物质是Mn3O4,因此反应的化学方程式为3MnO2![]() Mn3O4+O2↑。
Mn3O4+O2↑。

【题目】下列实验操作和所用装置能达到实验目的的是
选项 | A | B | C | D |
实验 目的 | 检验蔗糖与浓硫酸反应产物中有CO2 | 证明Fe(NO3)2溶液中存在Fe2+的水解平衡 | 检验某溶液中含有Ag+ | 比较非金属性:S>C>Si |
实验 操作 | 将浓硫酸滴入圆底烧瓶中,再将生成的气体通入澄清石灰水 | 将稀硝酸滴入Fe(NO3)2溶液中 | 向待检液中滴加足量的稀盐酸 | 将稀硫酸滴入锥形瓶中,再将生成的气体通入Na2SiO3溶液中 |
所用 装置 |
|
|
|
|
A. A B. B C. C D. D





