��Ŀ����
����Ŀ������ƽ������Һ��ѧ�е���Ҫ���ݣ�ij��ѧ��ȤС����������Ϊ��̽����������ʡ�
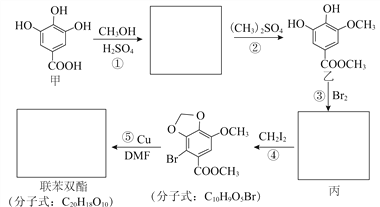
��1��ʵ��һ���������Ʊ���Һ�����ơ�
��Ҫ����0.1000mol/LNaOH����Һ250mL����Ҫ�õ��IJ��������в��������ձ�����ͷ�ιܺ�______���������������û�н��ձ��ȵ�ϴ��Һһ��ת������ƿ����������������ȷ������£����ñ���Һ�����ζ����У�2���е�δ֪Ũ�ȵ�������Һ������ʹ�ඨ�Ľ��ƫ�ߣ�����������������������Ӱ������
��2��ʵ���������һƿ������Һ�������²ⶨ��������ĵ���ƽ�ⳣ�������ʵ�鷽�����������������Ͷ�Ӧ�IJⶨ������д���±��С�
���������� | �ⶨ���� |
��_______ | ��ȡ25.00mL������Һ����ƿ�У��μ�ָʾ������0.1000mol/LNaOH����Һװ���ʽ�ζ��ܣ��ζ����յ㣬��¼���ݡ��ظ��ζ�2�Ρ� |
��H+�����ʵ���Ũ�� | ȡ����������Һ���ձ��У���______�ⶨ��ҺpH�� |
�� ����ʵ���У������ij�¶�ʱ���������Һ�����ʵ���Ũ��Ϊ0.1000mol/L��pH=3�����ڸ��¶�ʱ����ĵ���ƽ�ⳣ��Ϊ____________��
��3��ʵ������̽�����ǿ��������þ����Ӧ���ʵ�Ӱ�졣
�� ���ʵ�鷽�����±�������c=_______g��
��� | ������� | ���Ũ�ȣ�mol/L�� | ��������mL | þ������/g |
l | ���� | 0.5 | 17.0 | 2.0 |
2 | ���� | 0.5 | 17.0 | c |
�� ʵ�鲽�裺
a����ͼ��װ���У�������ҩƷ֮ǰ����________��
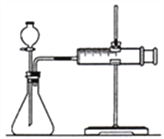
b����Ӧ��ʼ��__________��
c��������¼�����ݻ��Ƴ�����ͼ����ͼ����

�� д��þ��������Һ��Ӧ�����ӷ���ʽ��_______________��
�� ����O��5min������þ����Ӧ�ķ�Ӧ���ʱ仯���ɣ�_____________��
���𰸡� 250��������ƿ �� ������Һ�����ʵ���Ũ�� pH�ƻ���pH��ֽ 1��10-5 2.0 ���װ�õ������ԡ�ÿ��1min��¼һ������H2����� Mg+2CH3COOH=Mg2++2CH3COO- +H2�� ������þ����Ӧ��ʼ�η�Ӧ���ʺܿ죬һ��ʱ���Ӧ�������Լ�С
����������1����Ҫ����0.1000mol/LNaOH����Һ250mL����Ҫ�õ��IJ��������в��������ձ�����ͷ�ιܺ�250mL����ƿ���������������û�н��ձ��ȵ�ϴ��Һһ��ת������ƿ�������Ƶ�NaOHŨ��ƫ�ͣ���������������ȷ������£����ñ���Һ�����ζ�δ֪Ũ�ȵ�������Һ�����ı�Һ�����ƫ����ʹ�ඨ�Ľ��ƫ�ߣ�
��2������ĵ���̶����ѵ���ĵ���ʷ�����ռԭ���ܷ������İٷֱȣ���Ҫ�����ĵ���̶ȣ���Ӧ���������Һ��Ũ�Ⱥ���Һ��H+��Ũ�ȣ�
�����ݲⶨ������֪����Ϊ����к͵ζ���������NaOH��Һ�DZ�Һ���������Ǵ���Һ��ͨ���ζ����ɲ��������Һ�����ʵ���Ũ�ȣ����м�ҺӦʢ���ڼ�ʽ�ζ����У�
���������ܲ��������Һ��Ũ�ȣ��ʴ˲�ʵ���Ŀ���Dz�����Һ��H+��Ũ�ȣ������Ͼ�ȷ�IJ�����Һ��pH��Ӧ����pH�ƻ���pH��ֽ��������ȡ����������Һ���ձ��У���pH�ƻ���pH��ֽ�ⶨ��ҺpH��
������ĵ���ƽ�ⳣ��=![]() =
=![]() =1��10-5��
=1��10-5��
��3���� Ҫ̽�����ǿ��������þ����Ӧ���ʵ�Ӱ�죬����뱣������Ӱ�췴Ӧ���ʵ����ر���һ�£��ʴ���������Ũ��Ӧ��ͬ��þ������Ҳ��ͬ����c=2g��
�� a��װ�����Ӻ�������ҩƷ֮ǰ�������װ�������ԣ�
b����ͼ��֪����Ҫͨ����������ͬ��ʱ������ռ�������������Ĵ�С��������Ӧ���ʣ����ڷ�Ӧ��ʼ��Ӧÿ��1min��¼һ������H2���������
c���� þ��������Һ��Ӧ�����ӷ���ʽΪMg+2CH3COOH=Mg2++2CH3COO- +H2����
�� ����O��5min������þ����Ӧ�ķ�Ӧ���ʱ仯���ɣ�������þ����Ӧ��������ʱ��仯�����ԣ�������þ����Ӧ��ʼ�η�Ӧ���ʺܿ죬һ��ʱ���Ӧ�������Լ�С��

 ��У����ϵ�д�
��У����ϵ�д�