��Ŀ����
����Ŀ�����ֶ�����Ԫ��A��B��C��D��E��ԭ��������������A��Cͬ���壬B�� Dͬ���壬C���Ӻ�B���Ӿ�����ͬ�ĵ��Ӳ�ṹ��A��B��D��E�����γɹ����ͻ����A��B�γɵĻ�������ˮ�гʼ��ԣ�C��E�γɵĻ�������ˮ�г����ԡ��ش��������⣺
(1)����Ԫ���У�ԭ�Ӱ뾶������_____(��Ԫ�ط��ţ���
(2)д����Dͬ����Ԫ�ص��⻯���зе����{�����ʵ���ʽ_______��
(3)����E��ˮ��Ӧ�IJ����о���ǿ���������ʵĽṹʽ_______��
(4)A��E�γɵĻ�������A��B�γɵĻ����ﷴӦ�������д��ڵĻ�ѧ������Ϊ_______��
(5)C������ȼ�յIJ���Ͷ�뵽ˮ�з�����Ӧ�����ӷ���ʽΪ_______��
(6)��A��B��D��E���γɵĹ����ͻ������У����ȶ�����������������������ȼ�գ�д��ȼ�յĻ�ѧ����ʽ________ ��
���𰸡� Na ![]() H��O��Cl ���Ӽ����ۼ� 2Na2O2��2H2O=4Na����4OH����O2�� 2PH3��4O2=P2O5��3H2O
H��O��Cl ���Ӽ����ۼ� 2Na2O2��2H2O=4Na����4OH����O2�� 2PH3��4O2=P2O5��3H2O
�����������ֶ�����Ԫ��A��B��C��D��E��ԭ��������������A��B�γɵĹ��ۻ�������ˮ�гʼ��ԣ��û�����ΪNH3����AΪ��Ԫ�ء�BΪ��Ԫ�أ�A��Cͬ�壬C��ԭ���������ڵ�Ԫ�أ���CΪNaԪ�أ�B��Dͬ�壬��DΪ��Ԫ�أ�C��E�γɵĻ�������ˮ�г����ԣ���EΪClԪ�أ�
(1)ͬ�����������ԭ�Ӱ뾶��С��ͬ�������϶���ԭ�Ӱ뾶����NaԪ�ص�ԭ�Ӱ뾶���
(2)��Pͬ�����NԪ�ص��⻯��NH3������Ӽ���������е����{�������ʵ���ʽΪ![]() ��
��
(3)Cl2��ˮ��Ӧ����HCl��HClO������HClO����ǿ��������HClO�ĽṹʽΪH��O��Cl��
(4)A��E�γɵĻ�����HCl��A��B�γɵĻ�����NH3�����߷�Ӧ����NH4Cl��NH4Cl�����к��У����Ӽ������ۼ���
(5)Na������ȼ�����ɵ�Na2O2��ˮ������Ӧ�����ӷ���ʽΪ2Na2O2��2H2O=4Na����4OH����O2�� ��
(6)�ǽ�����Խ�����⻯��Խ���ȶ���N��P��Cl��PԪ�طǽ�����������PH3��ȶ�����������������ȼ�յĻ�ѧ����ʽΪ2PH3��4O2=P2O5��3H2O��

����Ŀ������ƽ������Һ��ѧ�е���Ҫ���ݣ�ij��ѧ��ȤС����������Ϊ��̽����������ʡ�
��1��ʵ��һ���������Ʊ���Һ�����ơ�
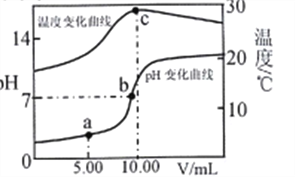
��Ҫ����0.1000mol/LNaOH����Һ250mL����Ҫ�õ��IJ��������в��������ձ�����ͷ�ιܺ�______���������������û�н��ձ��ȵ�ϴ��Һһ��ת������ƿ����������������ȷ������£����ñ���Һ�����ζ����У�2���е�δ֪Ũ�ȵ�������Һ������ʹ�ඨ�Ľ��ƫ�ߣ�����������������������Ӱ������
��2��ʵ���������һƿ������Һ�������²ⶨ��������ĵ���ƽ�ⳣ�������ʵ�鷽�����������������Ͷ�Ӧ�IJⶨ������д���±��С�
���������� | �ⶨ���� |
��_______ | ��ȡ25.00mL������Һ����ƿ�У��μ�ָʾ������0.1000mol/LNaOH����Һװ���ʽ�ζ��ܣ��ζ����յ㣬��¼���ݡ��ظ��ζ�2�Ρ� |
��H+�����ʵ���Ũ�� | ȡ����������Һ���ձ��У���______�ⶨ��ҺpH�� |
�� ����ʵ���У������ij�¶�ʱ���������Һ�����ʵ���Ũ��Ϊ0.1000mol/L��pH=3�����ڸ��¶�ʱ����ĵ���ƽ�ⳣ��Ϊ____________��
��3��ʵ������̽�����ǿ��������þ����Ӧ���ʵ�Ӱ�졣
�� ���ʵ�鷽�����±�������c=_______g��
��� | ������� | ���Ũ�ȣ�mol/L�� | ��������mL | þ������/g |
l | ���� | 0.5 | 17.0 | 2.0 |
2 | ���� | 0.5 | 17.0 | c |
�� ʵ�鲽�裺
a����ͼ��װ���У�������ҩƷ֮ǰ����________��
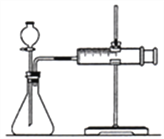
b����Ӧ��ʼ��__________��
c��������¼�����ݻ��Ƴ�����ͼ����ͼ����
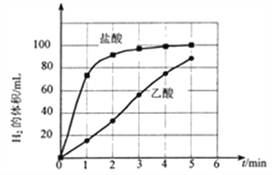
�� д��þ��������Һ��Ӧ�����ӷ���ʽ��_______________��
�� ����O��5min������þ����Ӧ�ķ�Ӧ���ʱ仯���ɣ�_____________��