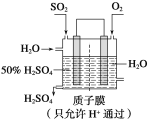
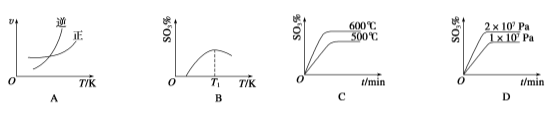
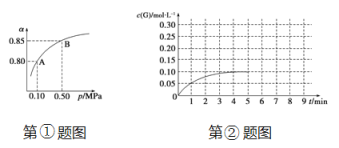
��Ŀ����
����Ŀ�������ʽṹ�����ʣ����һЩ�����ﳣ�����뵼�塢���ݼ���ɱ��ҩ�ȡ��ش��������⣺
��1����̬Asԭ�ӵĺ�������Ų�ʽΪ��Ar��________����________��δ�ɶԵ��ӡ�
��2���ڵ���Ͻ���ϵ�̫���ܵ��Ч�ʴ�40����Ga��N��As�縺���ɴ���С��˳����____________
��3�� As4O6�ķ��ӽṹ��ͼ��ʾ�� ����Asԭ�ӵ��ӻ���ʽΪ_________��1 molAs406�������������ʵ���Ϊ___________mol ��
����Asԭ�ӵ��ӻ���ʽΪ_________��1 molAs406�������������ʵ���Ϊ___________mol ��
��4��As��N��ͬ����Ԫ�أ�AsH3�ķе㣨-62.5������NH3�ķе㣨-33.5�����ͣ�ԭ����____________
��5��H3AsO4��H3AsO3��������ֺ����ᣬ���ݽṹ�����ʵĹ�ϵ��H3AsO4�����Ա�H3AsO3Asq ǿ��ԭ����_________________
��7����������������Ҫ��:
��ԭ�������������ʾ�����ڲ���ԭ�ӵ����λ�ã�LiZnAs��ϡ�Ű뵼��ľ�����ͼ��ʾ��

����ԭ���������A ��Li Ϊ(0,0��1/2)��B��AsΪ(1/4,1/4��1/4)��C��Li���������Ϊ__________��
���������������������Ĵ�С����״����֪LiZnAs �����ľ���������a ="594pm" , NA��ʾ�����ӵ���������ֵ�����ܶ�Ϊ__________g��cm-3���г�����ʽ���ɣ���
���𰸡���1��3d104s24p3��3��
��2��N>As>Ga����3��sp3��12��
��4��As��ԭ�Ӱ뾶��N�Ĵ縺�Ա�N��С��AsH3���Ӽ䲻���γ��������NH3���Ӽ����γ������
��5��H3AsO4��H3AsO3�ɷֱ��ʾΪ(HO)3AsO��(HO)3As��H3AsO3�е�AsΪ+3�ۣ���H3AsO4�е�AsΪ+5�ۣ������Ը��ߣ�����As-O-H��O�ĵ�����Asƫ�ƣ��������H+����
��6����![]() ����
����![]()
�������������������1��As��ԭ��������33������ݺ�������Ų����ɿ�֪��̬Asԭ�ӵĺ�������Ų�ʽΪ��Ar��3d104s24p3����3��δ�ɶԵ��ӡ�
��2���ǽ�����Խǿ�縺��Խ����Ga��N��As�縺���ɴ���С��˳����N>As>Ga��
��3������ As4O6�ķ��ӽṹʾ��ͼ��֪Asԭ�ӵļ۲���Ӷ�����4������As���ӻ���ʽΪsp3������������������1 molAs4O6�������������ʵ���Ϊ12mol��
( 4 ) ����As��ԭ�Ӱ뾶��N�Ĵ縺�Ա�N��С��AsH3���Ӽ䲻���γ��������NH3���Ӽ����γ����������AsH3�ķе㣨-62.5������NH3�ķе㣨-33.5�����͡�
��5�� H3AsO4��H3AsO3�ɷֱ��ʾΪ(HO)3AsO��(HO)3As��H3AsO3�е�AsΪ+3�ۣ���H3AsO4�е�AsΪ+5�ۣ������Ը��ߣ�����As-O-H��O�ĵ�����Asƫ�ƣ��������H+���ӣ����H3AsO4�����Ա�H3AsO3Asq ǿ��
��7�������ݾ����ṹ��֪C��Li���������Ϊ![]() ��
��
���þ�����Liԭ�ӵĸ�����![]() �����Ը��ݻ�ѧʽ��֪�þ������ܶȣ�
�����Ը��ݻ�ѧʽ��֪�þ������ܶȣ�![]() g��cm-3��
g��cm-3��

����Ŀ����2016�캪��һģ������ѧѡ��3�����ʽṹ�����ʡ�
���ʹ�������ǿ����������ܵ���Ҫ�о�����
��1��Ti(BH4)3��һ�ִ�����ϣ�����TiCl4��LiBH4��Ӧ�Ƶá�
����̬Clԭ���У�����ռ�ݵ�����ܲ����Ϊ_________�����ܲ���е�ԭ�ӹ����Ϊ________��
��LiBH4��Li+��BH4-���ɣ�BH4-������ṹ��_________��Bԭ�ӵ��ӻ����������________��
Li��B��HԪ�صĵ縺���ɴ�С����˳��Ϊ________��
��2�������⻯���Ǿ������÷�չǰ���Ĵ�����ϡ�
��LiH�У����Ӱ뾶Li+_______H-(������������=����������)����ij��������ǵ������ڽ���Ԫ��M���⻯�M�IJ��ֵ����������ʾ��
I1/kJmol-1 | I2/kJmol-1 | I3/kJmol-1 | I4/kJmol-1 | I5/kJmol-1 |
738 | 1451 | 7733 | 10540 | 13630 |
M��________ (��Ԫ�ط���)��
��3��NaH����NaCl�;���ṹ����֪NaH����ľ�������a=488pm(�ⳤ)��Na+�뾶Ϊ102pm��H-�İ뾶Ϊ________��NaH�������ܶ���___________g��cm-3(ֻ����ʽ�����ؼ������ֵ�������ӵ�����ΪNA)
����Ŀ����ȥ�������������������ʣ�ѡ�õ��Լ���ȷ���ǣ� ��
ѡ�� | ���ʣ����ʣ� | �Լ� |
A | Al2O3��SiO2�� | NaOH��Һ |
B | FeCl2��Һ��FeCl3�� | Fe�� |
C | CO2��SO2�� | Na2CO3��Һ |
D | NaHCO3��Һ��Na2CO3�� | Ca��OH��2��Һ |