��Ŀ����
����Ŀ��CO2��Դ�����õķ���֮һ�Ǻϳɶ����ѣ�CH3OCH3����
��1��CO2 ������ϳɶ����ѵĹ�������Ҫ�������з�Ӧ��
��ӦI�� CO2(g) + H2(g) ![]() CO(g) + H2O(g) H= + 41.2 kJ��mol1
CO(g) + H2O(g) H= + 41.2 kJ��mol1
��ӦII��2CO2(g) + 6H2(g) ![]() CH3OCH3(g) + 3H2O(g) H = ��122.5 kJ��mol1
CH3OCH3(g) + 3H2O(g) H = ��122.5 kJ��mol1
���У���ӦII�����¢١���������ɣ���д����Ӧ�ٵ��Ȼ�ѧ����ʽ����___________
�� 2CH3OH(g) ![]() CH3OCH3(g) + H2O(g) H = ��23.5 kJ��mol1
CH3OCH3(g) + H2O(g) H = ��23.5 kJ��mol1
��2��������ֱ��ȼ�ϵ�ؾ��������졢Ч�ʸߵ��ŵ㡣�������Ϊ���ԣ�������ֱ��ȼ�ϵ�صĸ�����ӦʽΪ____________���Ըõ��Ϊ��Դ���ö��Ե缫��ⱥ��ʳ��ˮ�Ļ�ѧ����ʽΪ________��
��3����ӦI������CO���������밴���з�Ӧ��ȥ��2CO(g) �� 2C(s) + O2(g)����֪�÷�Ӧ��H��0�������������ܷ�ʵ�ֵ�����_____________��
��4��CO2����Ȼ��ѭ��ʱ����CaCO3��Ӧ��Ksp��CaCO3��=2.8��109�� CaCl2��Һ��Na2CO3��Һ��Ͽ��γ�CaCO3�������ֽ��������CaCl2��Һ��Na2CO3��Һ��ϣ���Na2CO3��Һ��Ũ��Ϊ5.6��105mol��L1�������ɳ�������CaCl2��Һ����СŨ��Ϊ________��
���𰸡�CO2(g) + 3H2(g) CH3OH(g) + H2O(g)��H =-49.5 kJ��mol1 CH3OCH3+16OH--12e-=2CO32-+11H2O 2NaCl+2H2O![]() 2NaOH+Cl2��+H2�� �÷�Ӧ���������ؼ��ķ�Ӧ�����ݡ�G=��H-T��S��֪��G��0������ʵ�� 210-4mol/L
2NaOH+Cl2��+H2�� �÷�Ӧ���������ؼ��ķ�Ӧ�����ݡ�G=��H-T��S��֪��G��0������ʵ�� 210-4mol/L
��������
��1�������֪��ӦII�����¢١���������ɣ����ݸ�˹���ɣ�������ʽ��II-�ڣ�![]() �÷���ʽ�٣�CO2(g)+3H2(g)CH3OH(g)+H2O(g)��H=
�÷���ʽ�٣�CO2(g)+3H2(g)CH3OH(g)+H2O(g)��H=![]() kJ��mol1=-49.5kJ��mol1��
kJ��mol1=-49.5kJ��mol1��
��2���������Ϊ���ԣ�����������������ԭ��Ӧ��O2+2H2O+4e-=4OH-��������ֱ��ȼ�ϵ�صĸ�����ӦΪ������ʧ�������ɶ�����̼������Һ������̼���Σ����ԭ���غ�͵���غ㣬�����ĵ缫��ӦʽΪ��CH3OCH3+16OH--12e-=2CO32-+11H2O���ö��Ե缫��ⱥ��ʳ��ˮ�������������������������������������ƣ����Ļ�ѧ����ʽΪ��2NaCl+2H2O![]() 2NaOH+Cl2��+H2����
2NaOH+Cl2��+H2����
��3��2CO(g)��2C(s)+O2(g)H��0���÷�Ӧ���������ؼ��ķ�Ӧ�����ݡ�G=��H-T��S��֪��G��0������ʵ�֣�
��4��Na2CO3��Һ��Ũ��Ϊ5.6��105mol��L1���������Ϻ�Ũ�ȼ�Сһ���Ϊ2.8��105mol��L1����Ksp(CaCO3)=c(CO32-)c(Ca2+)��֪����Ϻ�c(Ca2+)=![]() =
=![]() =110-4mol/L����������CaCl2��Һ����СŨ��Ϊ110-4mol/L2=210-4mol/L��
=110-4mol/L����������CaCl2��Һ����СŨ��Ϊ110-4mol/L2=210-4mol/L��

 ��У����ϵ�д�
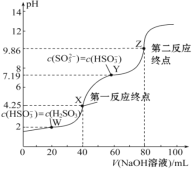
��У����ϵ�д�����Ŀ��ijͬѧ����Ӱ�������Һ��������������Һ��Ӧ�������ص��о��������£�ʵ���������£�
ʵ����� | �� | �� | �� |
�����Լ� | 4mL 0.01mol/L KMnO4 2mL 0.1mol/L H2C2O4 | 4mL 0.01mol/L KMnO4 2mL 0.1mol/L H2C2O4 ����MnSO4���� | 4mL 0.01mol/L KMnO4 2mL 0.1mol/L H2C2O4 ����Na2SO4���� |
��ɫʱ��/s | 116 | 6 | 117 |
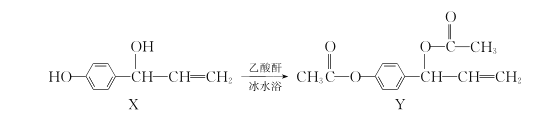
��1��������Һ��������������Һ��Ӧ�����ӷ���ʽΪ___________________��
��2����ʵ�������_______________________��
��3��������������ʵ���ͬѧ���з�˼����Ϊʵ��ٵ��������֤���������ۡ���д��ʵ��ٵ�����Ϊ____________________��
��4��ʵ���ѡ��MnSO4���������MnCl2�����ԭ����_________________��
��5����ͬѧ���������ͼ��ʾ��ʵ�鷽������̽����������Է�Ӧ���ʵ�Ӱ�졣

a����ͬѧ���о���Ӱ��������___________________��
b������Ϊ��ͬѧ��ʵ�鷽��_______________���������������������������____________________________��