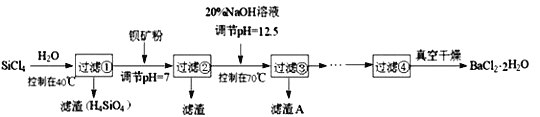
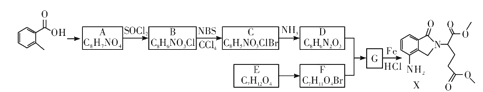
��Ŀ����
����Ŀ�����ױ�������ҵ�ϣ��������м��ͨ���Ȼ���������ˮ�Ȼ�����;��ͬ�����£������(C2O42-)�Ļ�ԭ��ǿ��Fe2+��Ϊ������һ���ۣ�������ѧ��ѧ�о���С���������ʵ�飺
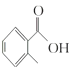
����:i. ����(H2C2O4)Ϊ��Ԫ���ᡣ
ii. ��ˮ������������[K3Fe(C2O4)33H2O]Ϊ����ɫ���壬�����ֽ⡣��ˮ��Һ�д���[Fe(C2O4)3]3-![]() Fe3++3C2O42- K=6.3��10-21
Fe3++3C2O42- K=6.3��10-21
iii.FeC2O42H2OΪ��ɫ���壬����ˮ��������ǿ�ᡣ
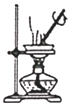
��ʵ��1��������װ����ȡ��ˮ�Ȼ�����
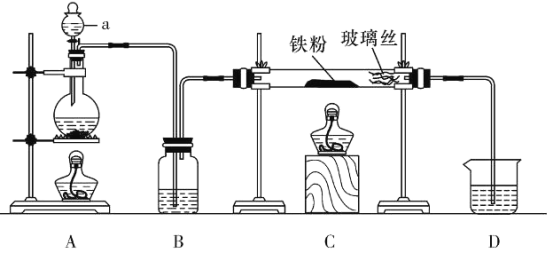
(1)����a������Ϊ___________��
(2)���Ƶô�����FeCl2��ʵ������е�ȼA��C�ƾ��Ƶ��Ⱥ�˳����___________��
(3)����D��װ�ý���β�����������ڵ�������__________��___________��
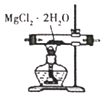
��ʵ��2��ͨ��Fe3+��C2O42-����Һ�еķ�Ӧ�Ƚ�Fe2+��C2O42-�Ļ�ԭ��ǿ����

(4)ȡʵ��2����������ϴ���������Һ�����KSCN��Һ������졣�����������ᣬ��Һ��죬˵�������к���+3�۵���Ԫ�ء����������Һ����ԭ����______________��
(5)�����飬����ɫ����ΪK3Fe(C2O4)33H2O�����ʵ�飬ȷ��ʵ��2��û�з���������ԭ��Ӧ�IJ�����������_____��
(6)ȡʵ��2�еĴ���ɫ��Һ����һ��ʱ�䣬������ɫ�����������ݲ�������ȫ��Ӧ�����ӷ���ʽ��_____Fe(C2O4)3]3-+____H2O![]() ____FeC2O4��2H2O��+__________+_______
____FeC2O4��2H2O��+__________+_______
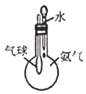
��ʵ��3���о���С�����������װ��ֱ�ӱȽ�Fe2+��C2O42-�Ļ�ԭ��ǿ�������ﵽ��Ԥ�ڵ�Ŀ�ġ�
(7)�����ﵽ��Ŀ�Ŀ��ܲ���������_____________________��
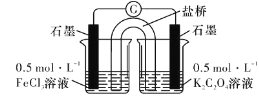
���𰸡���Һ©�� �ȵ�ȼA���ƾ��ƣ��ٵ�ȼC���ƾ��� �������� ��ȼ������H2���ܱ����� ��Һ�д���ƽ�⣺[Fe(C2O4)3]3-![]() Fe3++3C2O42-�����������H+��C2O42-��Ͽ�ʹƽ�������ƶ���c(Fe3+)������KSCN��Һ��� ȡ����ʵ��2�еĴ���ɫ��Һ���μ�K3[Fe(CN)6]��Һ����������ɫ���� 2 4 2 3C2O42- 2CO2�� �����Ƶ�ָ�뷢��ƫת��һ��ʱ��������Һ��Ϊdz��ɫ���Ҳ������ݲ���
Fe3++3C2O42-�����������H+��C2O42-��Ͽ�ʹƽ�������ƶ���c(Fe3+)������KSCN��Һ��� ȡ����ʵ��2�еĴ���ɫ��Һ���μ�K3[Fe(CN)6]��Һ����������ɫ���� 2 4 2 3C2O42- 2CO2�� �����Ƶ�ָ�뷢��ƫת��һ��ʱ��������Һ��Ϊdz��ɫ���Ҳ������ݲ���
��������
ʵ��һ����װ��A����Ũ������NaCl�����ϼ�����ȡHCl��ͨ��Bװ�õ�Ũ�������ô���HCl���壬Ȼ����װ��C��Fe��HCl������Ӧ����FeCl2��H2����Ӧ��������к�H2��δ��Ӧ��HCl���壬�ɸ���HCl����������ˮ����ˮ�����ռ����ս���β��������
ʵ�����FeCl3��Һ��K2C2O4�������ֽⷴӦ����Fe2(C2O4)3��KCl����KSCN��Һ����Fe3+����K3[Fe(CN)6]��Һ����Fe2+�����ݵ����غ㡢����غ㼰ԭ���غ���д���ӷ���ʽ��
ʵ����������2Fe3++C2O42-=2Fe2++2CO2�����ж�ԭ�����������Ӧ����Ӧ������
(1)����ͼʾ��֪����a�����Ƿ�Һ©����
(2)Ϊ��ֹFe��װ���еĿ���������Ӧ���Ƶô�����FeCl2��ʵ��������ȵ�ȼA���ƾ��ƣ�ʹװ�ó���HCl���壬Ȼ���C���ƾ��Ƽ��ȣ�
(3)����D��װ�ý���β������������HCl����������ˮ��HCl�ܽ�µ�����������ѹǿ��С������������ķ��������ҿ�ȼ������H2���ܱ����գ�
(4)��ʵ����У���FeCl3��Һ�м���K2C2O4��Һ���������ֽⷴӦ����Fe2(C2O4)3��KCl�������Ĵ���ɫ����ΪFe2(C2O4)3�Ľᾧˮ����K3Fe(C2O4)33H2O��ȡʵ��2����������ϴ���������Һ�����KSCN��Һ������죬�����������ᣬ��Һ��죬˵�������к���+3�۵���Ԫ�ء�����������Һ��t��ԭ��������Һ�д��ڵ���ƽ�⣺��Һ�д���ƽ�⣺[Fe(C2O4)3]3-![]() Fe3++3C2O42-���������������������H+����Һ�е�C2O42-�����������H2C2O4��ʹ��ʹƽ�������ƶ���������Һ��c(Fe3+)������KSCN��Һ��죻
Fe3++3C2O42-���������������������H+����Һ�е�C2O42-�����������H2C2O4��ʹ��ʹƽ�������ƶ���������Һ��c(Fe3+)������KSCN��Һ��죻
(5)��K3Fe(C2O4)33H2O����������ԭ��Ӧ��������Fe2+�����鷽����ȡ����ʵ��2�еĴ���ɫ��Һ���μ�K3[Fe(CN)6]��Һ����������ɫ������֤����Fe2+��������K3Fe(C2O4)33H2Oδ����������ԭ��Ӧ��
(6)�ڹ��������²�������Һ����������ԭ��Ӧ������FeC2O4��2H2O��CO2���壬���ݵ����غ㡢����غ㼰ԭ���غ㣬�ɵ÷�Ӧ�����ӷ���ʽ��2Fe(C2O4)3]3-+4H2O![]() 2FeC2O4��2H2O��+2C2O42��+2CO2����
2FeC2O4��2H2O��+2C2O42��+2CO2����
(7)��װ�ù�����ԭ��أ�����ߣ�Fe3+��õ��ӱ�ΪFe2+����Һ��Ϊdz��ɫ����ߵ缫Ϊ���������ұߵ缫�ϣ���Һ�е�C2O42-ʧȥ���ӣ�����������Ӧ��C2O42--2e-=2CO2�����ұߵ缫Ϊ�������ῴ���缫�������ݲ�����

 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�����Ŀ������������KMnO4��H2C2O4(����)��Ӧ�о�Ӱ�췴Ӧ���ʵ����أ����ӷ���ʽΪ2 MnO4-��5H2C2O4��6H+ = 2Mn2+��10CO2����8H2O��һʵ��С����ͨ���ⶨ��λʱ��������CO2�����ʣ�̽��ij��Ӱ�컯ѧ��Ӧ���ʵ����أ����ʵ�鷽������(KMnO4��Һ���ữ)��ʵ��װ����ͼ����ʾ��

ʵ����� | A��Һ | B��Һ |
�� | 20 mL 0.1 mol��L��1 H2C2O4��Һ | 30 mL 0.01 mol��L��1 KMnO4��Һ |
�� | 20 mL 0.2 mol��L��1 H2C2O4��Һ | 30 mL 0.01 mol��L��1 KMnO4��Һ |
(1)��ʵ��̽������_________________________________���ضԻ�ѧ��Ӧ���ʵ�Ӱ�졣
(2)��ʵ�����2 minĩ�ռ���4.48 mL CO2(��״����)������2 minĩ��c(MnO4-)��________ mol��L��1(��������Һ�����Ϊ50 mL)��
(3)С��ͬѧ���ַ�Ӧ���ʱ仯��ͼ�ң�����t1��t2ʱ�������ʱ�����Ҫԭ������ǣ�
�ٲ���Mn2���Ƿ�Ӧ�Ĵ�������_______________________________
���¶ȸ���500 Kʱ����ѧ�ҳɹ����ö�����̼�������ϳ����Ҵ������ڽ��ܼ��š�����̼�ŷŷ�������ش����塣�ش��������⣺
(1)�÷�Ӧ�Ļ�ѧ����ʽΪ___________________________________
(2)�ں��º����ܱ������У��ж�������Ӧ�ﵽƽ��״̬��������________________
a����ϵѹǿ���ٸı� b��H2��Ũ�Ȳ��ٸı�
c��������ܶȲ���ʱ��ı� d����λʱ��������H2��CO2�����ʵ���֮��Ϊ3��1
����Ŀ���������ף�����Ũ����ͻ��ý�����Ӧ�����У���������Ũ�ȵĽ��ͣ������ɵIJ�������4����2����3�۵��Ļ����
��FeSO4 + NO![]() Fe(NO)SO4(��ɫ)����H��0��
Fe(NO)SO4(��ɫ)����H��0��
��NO2��NO���ܱ�����KMnO4��Һ�������ա�
�װ�����ͼ��ʾ��ʵ��װ�ý���ʵ�飺
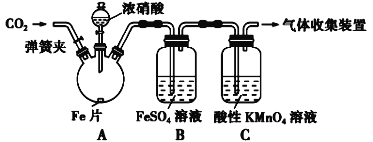
ʵ������������¼�����ʾ��
ʵ����� | ʵ������ |
���ɼУ�ͨ��һ��ʱ��CO2���رյ��ɼ� | |
��Һ©����������Ũ���Ỻ��������ƿ�У��رջ��� | ���������� |
������ƿ����Ӧ��ʼ��ֹͣ���� | ��A���к���ɫ���������һ��ʱ���������ɫ��dz��B����Һ����ɫ��C����Һ��ɫ��dz�� �ڷ�Ӧֹͣ��A������ʣ�� |
��ش��������⣺
�� ����ǰ������ƿ�е���Ũ����û�����������ԭ����________________________________
�� �����Ƿ����ɣ�3�۵��Ļ����Ӧ���е�ʵ�������________________________________
�� ��ȡ����B����Һ�����ȣ�ʵ��������____________________________________________�����û�ѧƽ��ԭ������ԭ��________________________________________________________�������ݸ�����ó����ۣ��������ᷴӦ��NO���ɡ�
������Ϊ�ó�A����NO���ɵ�֤�ݲ��㡣Ϊ��ȡ�����֤�ݣ����Բ��ø�װ�úͲ������ж���ʵ�飬�������ĸı���____________________________��֤����NO���ɵ�ʵ��������________________________________________________