��Ŀ����
8����1���±�ʾԪ�����ڱ���һ����| ��A | ��A | ��A | ��A | ��A | ��A | ��A | 0 | |
| 2 | a | b | ||||||
| 3 | c | d | e | f | g | h | i |
��c��d��e��b��ԭ�Ӱ뾶�ɴ�С��˳����Na��Mg��Al��F��
��g��h������������ˮ����������ǿ����HClO4��
��a��f���⻯�����ȶ���ǿ����SiH4��
��aԪ�غ���Ԫ���γɵĻ�����ĵ���ʽ��
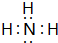 ��
����cԪ�غ�bԪ���γɵĻ������к��еĻ�ѧ�����������Ӽ���
��2���±���Ԫ�����ڱ���һ��Ƭ�Σ���Ԫ�ؾ����ڶ�����Ԫ��
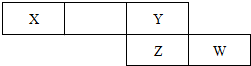
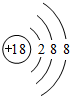
����X�ĵ���Ϊ��������Ҫ�ɷ�֮һ����W��ԭ�ӽṹʾ��ͼ
 ��
������Y�������γɷ�Ӧ���ɵ���ɫ���壬��õ���ɫ����Ļ�ѧʽΪNa2O2�����еĻ�ѧ�����������Ӽ������ۼ���
���� ��1������Ԫ�������ڱ���λ�ÿ�֪��aΪN��bΪF��cΪNa��dΪMg��eΪAl��fΪSi��gΪS��hΪCl��iΪAr��
��ͬ�����������ԭ�Ӱ뾶��С��ͬ�������϶���ԭ�Ӱ뾶����
�۷ǽ�����Խǿ����ۺ����������Խǿ��
��Ԫ�طǽ�����Խǿ����Ӧ�⻯��Խ�ȶ���
��aԪ�غ���Ԫ���γɵĻ�����ΪNH3��
��cԪ�غ�bԪ���γɵĻ�����ΪNaF��
��2������X�ĵ���Ϊ��������Ҫ�ɷ�֮һ����XΪN��YΪF��ZΪCl��WΪAr��
����Y�������γɷ�Ӧ���ɵ���ɫ���壬��YΪO��XΪC��ZΪS��WΪCl��
��� �⣺��1������Ԫ�������ڱ���λ�ÿ�֪��aΪN��bΪF��cΪNa��dΪMg��eΪAl��fΪSi��gΪS��hΪCl��iΪAr���ʴ�Ϊ��Si��Cl��
��ͬ�����������ԭ�Ӱ뾶��С��ͬ�������϶���ԭ�Ӱ뾶����ԭ�Ӱ뾶��Na��Mg��Al��F���ʴ�Ϊ��Na��Mg��Al��F��
�۷ǽ�����Cl��S���ǽ�����Խǿ����ۺ����������Խǿ�������ԣ�HClO4��H2SO4���ʴ�Ϊ��HClO4��
�ܷǽ�����N��Si��Ԫ�طǽ�����Խǿ����Ӧ�⻯��Խ�ȶ������⻯���ȶ��ԣ�NH3��SiH4���ʴ�Ϊ��SiH4��
��aԪ�غ���Ԫ���γɵĻ�����ΪNH3������ʽΪ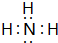 ���ʴ�Ϊ��
���ʴ�Ϊ��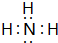 ��
��
��cԪ�غ�bԪ���γɵĻ�����ΪNaF���������Ӽ����ʴ�Ϊ�����Ӽ���
��2������X�ĵ���Ϊ��������Ҫ�ɷ�֮һ����XΪN��YΪF��ZΪCl��WΪAr��W��ԭ�ӽṹʾ��ͼΪ ���ʴ�Ϊ��
���ʴ�Ϊ�� ��
��
����Y�������γɷ�Ӧ���ɵ���ɫ���壬��YΪO��XΪC��ZΪS��WΪCl���õ���ɫ����Ļ�ѧʽΪNa2O2���������Ӽ������ۼ����ʴ�Ϊ��Na2O2�����Ӽ������ۼ���
���� ���⿼��Ԫ�����ڱ���Ԫ�������ɣ��Ƚϻ�����ע���Ԫ�����ڱ���Ԫ�������ɵ��������գ�

 ���Ǽ���С����ϵ�д�
���Ǽ���С����ϵ�д� �Ͻ�ƽ���Ȿϵ�д�
�Ͻ�ƽ���Ȿϵ�д�| A�� |  | B�� |  | C�� |  | D�� |  |
| A�� | ȼ�����ڷ��ȷ�Ӧ | |
| B�� | �кͷ�Ӧ�������ȷ�Ӧ | |
| C�� | ��ѧ������Ҫ�ͷ����� | |
| D�� | ��Ӧ����������������������һ����� |
| A�� | F | B�� | O2 | C�� | CH4 | D�� | C2H6 |

| A�� | X�������ȶ� | B�� | Y���⻯��ΪHY | ||
| C�� | Z���ʳ������ǵ���ɫ���� | D�� | ������HXO |
| A�� | BaCl2��Һ��K2SO4��Һ��Ӧ | B�� | Cl2ͨ��NaOH��Һ�� | ||
| C�� | NaOH��Һ��CuSO4��Һ��Ӧ | D�� | Cl2ͨ��NaI��Һ�� |
| A�� | KCl | B�� | CO2 | C�� | CaO | D�� | C6H12O6 |
| A�� | �� | B�� | �� | C�� | �� | D�� | �� |