��Ŀ����
18�����ǵؿ��к�����Ϊ�ḻ�ķǽ���Ԫ�أ���Ҫ��������ˮ����������Ca3��PO4��2����ʽ���ڣ����ĵ��ʺͻ������ڹ�ũҵ������������Ҫ��Ӧ�ã���1�����ף�P4������Ca3��PO4��2����̿��SiO2�ڵ�¯�и��£���1550�棩��ͨ������������Ӧ���۵õ���
��4Ca3��PO4��2��s��+10C��s���T12CaO��s��+2P4��s��+10CO2��g����H1=+Q1kJ•mol-1
��CaO��s��+SiO2��s���TCaSiO3��s����H2=-Q2 kJ•mol-1
��CO2��g��+C��s���T2CO��g����H3=+Q3kJ•mol-1
��֪��CaSiO3���۵㣨1546�棩��SiO2�ͣ�
д��������ƿ���ȡ�����ܵķ�Ӧ����ʽ2Ca3��PO4��2+6SiO2+10C$\frac{\underline{\;����\;}}{\;}$6CaSiO3+P4+10CO��
��2���������ȵ�Ũ����������Һ���绯�õ�һ�ִ������Σ�KH2PO2����һ������PH3��д��ѧʽ����
��3������Ҫ������NaH2PO4��ͨ��H3PO4��NaOH��Һ��Ӧ��ã���ҵ��Ϊ��ʹ��Ӧ����Ҫ������NaH2PO4��ͨ����pH������2.1��7.2֮�� ����֪����ĸ������볣��Ϊ��K1=7.1��10-3K2=6.3��10-8K3=4.2��10-13 lg7.1��0.9 lg6.3��0.8 lg4.2��0.6����Na2HPO4 ��Һ�Լ��ԣ���������Һ�м���������CaCl2 ��Һ����Һ�������ԣ���ԭ����3Ca2++2HPO4-=Ca3��PO4 ��2��+2H+ �������ӷ���ʽ��ʾ����
��4�������ж������CuSO4��Һ�ⶾ���ⶾԭ���������л�ѧ����ʽ��ʾ��11P 4+60CuSO4+96H2O�T20Cu3P+24H3PO4+60H2SO460molCuSO4�������������ʵ�����3mol��
���� ��1�������Ȼ�ѧ����ʽ��˹���ɼ������軯ѧ����ʽ��
��2���绯��Ӧ��Ԫ������Ϊ�������Σ���Ԫ�ػ��ϼ�Ϊ+1�ۣ����͵�ֻ��Ϊ���ۣ���Ϊ�⻯�
��3��H3PO4?H++H2PO4-K1=7.1��10-3��H2PO4-?H++HPO42-K2=6.3��10-8��HPO42-?H++HPO43-K3=4.2��10-13����һ�����������H+����һ���������������ã�Ϊ��þ����ܴ���NaH2PO4����H2PO4-Ũ�������K1��K2���Լ�pH=-lgc��H+�����㣻
����Na2HPO4��Һ�м���������CaCl2��Һ��HPO42-���Ӻ�Ca2+���ӷ�Ӧ���ɳ������ٽ�HPO42-�ĵ��룬��Һ��ʾ���ԣ�
��4��CuԪ�صĻ��ϼ���+2�۽��͵�+1�ۣ�CuSO4����������P4������Ԫ����0�۽��͵�-3�ۣ�������Ԫ����0�����ߵ�+5�ۣ���Ԫ�صĻ��ϼۼ������ֽ��ͣ�����P4�������������ǻ�ԭ��������11molP4�μӷ�Ӧ������5mol��P4����������60mol����ͭ����������ֻ��6mol��P4����ԭ������ϵ����غ������
��� �⣺��1����4Ca3��PO4��2��s��+10C��s��=12CaO��s��+2P4��s��+10CO2��g����H1=+Q1kJ•mol-1
��CaO��s��+SiO2��s��=CaSiO3��s����H2=-Q2 kJ•mol-1
��CO2��g��+C��s��=2CO��g����H3=+Q3kJ•mol-1
�����Ȼ�ѧ����ʽ��˹���ɼ���õ��١�$\frac{1}{2}$+��+�ڡ�6�õ���ѧ����ʽΪ��2Ca3��PO4��2+6SiO2+10C$\frac{\underline{\;����\;}}{\;}$6CaSiO3+P4+10CO��
�ʴ�Ϊ��2Ca3��PO4��2+6SiO2+10C$\frac{\underline{\;����\;}}{\;}$6CaSiO3+P4+10CO��
��2���������ȵ�Ũ����������Һ��᪻��õ�һ�ִ������Σ�KH2PO2����һ�����壬�绯��Ӧ��Ԫ������Ϊ�������Σ���Ԫ�ػ��ϼ�Ϊ+1�ۣ����͵�ֻ��Ϊ���ۣ���Ϊ�⻯��PH3��
�ʴ�Ϊ��PH3��
��3��Ϊ��þ����ܴ���NaH2PO4�����������ᡢ��������ƵĻ�����Һ������Һȫ��Ϊ������Һʱ�������Ե�һ������Ϊ��������H3PO4?H++H2PO4-K1=7.1��10-3��PH=-lgc��H+��=3-lg7.1��2.1��
����Һȫ��ΪNaH2PO4��Һʱ��H2PO4-?H++HPO42-K2=6.3��10-8������pH=-lgc��H+��=8-lg6.3��7.2��
����pHӦ���ƽ���2.1��7.2֮�䣬
HPO42-���Ӽ��ܷ����������ܷ���ˮ�⣬���뷴ӦʽΪHPO42-?PO43-+H+��ˮ�ⷴӦʽΪHPO42-+H2O?H2PO4-+OH-����Һ�ʼ��ԣ�˵��ˮ��̶ȴ��ڵ���̶ȣ�
����HPO42-���Ӻ�Ca2+���ӷ�Ӧ���ɳ�����3Ca2++2HPO42-�TCa3��PO4��2��+2H+���ٽ�HPO42-�ĵ��룬��Һ�������ԣ�
�ʴ�Ϊ��2.1��7.2��3Ca2++2HPO4-=Ca3��PO4 ��2��+2H+��
��4��CuԪ�صĻ��ϼ���+2�۽��͵�+1�ۣ�CuSO4����������P4������Ԫ����0�۽��͵�-3�ۣ�������Ԫ����0�����ߵ�+5�ۣ���Ԫ�صĻ��ϼۼ������ֽ��ͣ�����P4�������������ǻ�ԭ��������11molP4�μӷ�Ӧ������5mol��P4����������60mol����ͭ����������ֻ��6mol��P4����ԭ����
���ɵ����غ��֪����60mol��CuSO4�μӷ�Ӧ��60molCuSO4�õ�60mol���ӣ�1molP4�μӷ�Ӧʧȥ20mol���ӣ�����60molCuSO4�������������ʵ�����$\frac{60}{20}$=3mol��
�ʴ�Ϊ��3mol��
���� ���⿼������ϵĶ����жϺͼ��㡢��˹���ɵ�Ӧ�á�������ԭ��Ӧ����ת�Ƽ��㡢���ӷ���ʽ����д�ȣ���Ŀ�漰��֪ʶ��϶࣬������ѧ���ķ��������ͼ��������Ŀ��飬��Ŀ�Ѷ��еȣ�

| A�� | K+��SiO32-��Cl-��NO3- | B�� | Cu2+��NH4+��Al3+��SO42- | ||
| C�� | Na+��S2-��OH-��SO42- | D�� | Na+��ClO-��CH3COO-��HCO3- |
| ��������� | ���� |
| ���������Ũ�ȵ�AgNO3��Һ��NaCl��Һ��ϵõ���ҺW�����ˣ��õ���ҺX�Ͱ�ɫ����Y | |
| ������ҺX�еμӼ��α���Na2S��Һ | ���ֻ��� |
| ��ȡ������ɫ����Y���μӼ��α���Na2S��Һ | ������Ϊ��ɫ |
| ����ȡ������ɫ����Y���μӼ���Ũ��ˮ | �������ܽ� |
 Ag+��aq��+Cl-��aq����
Ag+��aq��+Cl-��aq������2���ɲ����Ļ��ǿ��Ʋ⣬��ҺX�г��˺���Na+��NO3-�������е�������Ag+��Cl-��
��3����˵��������г�����ڵ����ӷ���ʽΪ2AgCl��s��+S2-�TAg2S��s��+2Cl-������ת������Ҫԭ���Ǹ��¶��£�Ag2S��AgCl�ܽ�ȸ�С��
��4����֪��Ag++2NH3•H2O Ag��NH3��2++2H2O����ƽ���ƶ�ԭ�����Ͳ�����м���Ũ��ˮ�������ܽ��ԭ��AgCl��g��
 Ag+��aq��+Cl-��aq�����������백ˮ��ϣ���������Һ�������ӵ�Ũ�ȣ�ʹ����ƽ�������ƶ�����ʹAgCl�ܽ⣮
Ag+��aq��+Cl-��aq�����������백ˮ��ϣ���������Һ�������ӵ�Ũ�ȣ�ʹ����ƽ�������ƶ�����ʹAgCl�ܽ⣮��5�����������Ϣ����������Ԥ�ⲻ��ȷ����BC��
A���ڲ����֮�����μ�Ũ���������AgCl��������
B���ɲ���������Ʋ⣺ʵ���ҿ��ð�ˮϴ��������Ӧ����Թ�
C�����ڰ�ɫ����Y�еμ�NaOH��Һ������Ҳ���ܽ⣮
| A�� | 100mL��0.01mol/L��CH3COOH��Һ��10mL��0.1mol/L��CH3COOH��Һ��H+��Ŀ | |
| B�� | ������pH=4��KHSO4��Һ��CH3COOK��Һ����ˮ�������OH-����Ũ�� | |
| C�� | ��ˮ��100��ʱ��pH��25��ʱ��pH | |
| D�� | 100mL��0.01mol/L��CH3COOH��Һ��10mL��0.1mol/L��CH3COOH��Һ��H+Ũ�� |
| A�� | 2 | B�� | 3 | C�� | 4 | D�� | 5 |
| A�� | 1mol����ϩ����4mol H2�����ӳɷ�Ӧ��˵��������ں���4��̼̼˫�� | |
| B�� | ��ŨNa2SO4��CuSO4��Һ��Ũ������Һʹ����Һ�����������������롢�ᴿ������ | |
| C�� | ����ˮ������ά��ˮ��ʱ�����ɵ����ղ��ﲻ��ͬ | |
| D�� | ��֬������NaOH��Һ����������Ӧ |
| ��A | ��A | ��A | ��A | ��A | ��A | ��A | 0 | |
| 2 | a | b | ||||||
| 3 | c | d | e | f | g | h | i |
��c��d��e��b��ԭ�Ӱ뾶�ɴ�С��˳����Na��Mg��Al��F��
��g��h������������ˮ����������ǿ����HClO4��
��a��f���⻯�����ȶ���ǿ����SiH4��
��aԪ�غ���Ԫ���γɵĻ�����ĵ���ʽ��
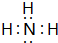 ��
����cԪ�غ�bԪ���γɵĻ������к��еĻ�ѧ�����������Ӽ���
��2���±���Ԫ�����ڱ���һ��Ƭ�Σ���Ԫ�ؾ����ڶ�����Ԫ��
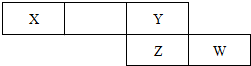
����X�ĵ���Ϊ��������Ҫ�ɷ�֮һ����W��ԭ�ӽṹʾ��ͼ
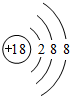 ��
������Y�������γɷ�Ӧ���ɵ���ɫ���壬��õ���ɫ����Ļ�ѧʽΪNa2O2�����еĻ�ѧ�����������Ӽ������ۼ���

