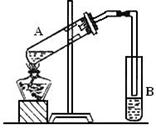
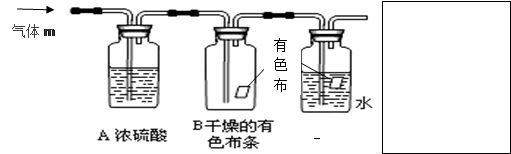
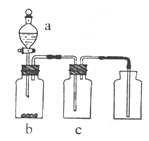
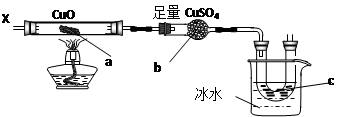
��Ŀ����
��4�֣�ȡ����п�ۣ���ʢ�ڼס��ҡ�����֧�Թ��У��ֱ�����������ʺ�ʱ���ϵ������ӣ���ַ�Ӧ������50mLpH=2���ᣬ�Ҽ���50mL pH=2���ᣬ������50mL pH=2���ἰ�����ĵ�����ĩ��
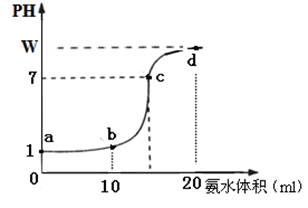
��1������Ӧ���˼����в������������һ���࣬��ֻ��һ֧�Թ��н�����ʣ�࣬��ʼʱ�����п��������С��ϵΪ �������á��ס��ҡ��� ��> �� < �� ="��" �ش�
��2������Ӧ�����������������һ���࣬��û��ʣ���п���ش��������⡣
����֧�Թ��вμӷ�Ӧ��п��������С��ϵΪ��
���á��ס��ҡ������� ��> �� < �� ="��" �ش�
�ڷ�Ӧ���ˣ�����ʱ��Ϊ�� ��ͬ�٣�
������������ʵ�飬п�۾�������������������� ��ͬ�٣�
��1������Ӧ���˼����в������������һ���࣬��ֻ��һ֧�Թ��н�����ʣ�࣬��ʼʱ�����п��������С��ϵΪ �������á��ס��ҡ��� ��> �� < �� ="��" �ش�
��2������Ӧ�����������������һ���࣬��û��ʣ���п���ش��������⡣
����֧�Թ��вμӷ�Ӧ��п��������С��ϵΪ��
���á��ס��ҡ������� ��> �� < �� ="��" �ش�
�ڷ�Ӧ���ˣ�����ʱ��Ϊ�� ��ͬ�٣�
������������ʵ�飬п�۾�������������������� ��ͬ�٣�
��4�֣���1����>�� (2)�ٱ�>��=�� �ڼ�>��>�� ����=��>��
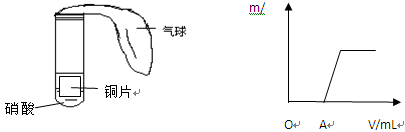
��1�������������ᣬ��Һ�еĴ��Ჿ�ֵ��룬��Һ�в����������Դ�����ӵ���ʽ���ڣ����������ͬ��PH��ͬ�Ĵ�������ᣬ����ʵ�ʺ��е������ӵ����ʵ�����������ʵ�ʺ��е������ӵ����ʵ�����������ֻ�Թܲ������������һ���࣬˵������û�з�Ӧ��ȫ�������п�۲��㣻ֻ��һ֧�Թ��н�����ʣ�࣬��ôֻ����������Թ��н���ʣ�࣬����Ǵ�����Թ��н���ʣ����ô����������һ�����ҹܶ࣬���Կ�ʼʱ�����п��������С��ϵΪ��>�ҡ�
��2���ٷ�Ӧ�����������������һ���࣬������ֻ��п�����ᷴӦ�����ɵ�����һ������ݵ�ʧ�����غ�����ж����ĵ�п��ҲӦ��һ���࣬��=�ң���֧�Թ���п�۾�û��ʣ�࣬�����к�������ͭ��Һ����п�۷�Ӧ����п�ۣ����Ա��м����п�۱Ƚ϶࣬���Լ���п�۵�������СΪ��>��=�ҡ��ڱ��д�������ͭ��п�����䷴Ӧ����ͭ�������γ�ԭ��أ����Է�Ӧ��죬����ʱ����̣��������ŷ�Ӧ�Ľ�����Һ�еĴ�����ӵ���ƽ�������ķ�����У���Һ�е������ӵõ����ʵ��IJ��䣬�����Թ��������Ӳ�������Ũ�ȼ�С��Ӧ���ʼ�С����Ӧ����������ʱ��������Է�Ӧ�����ʱ��Ϊ��>��>������п�۹�����������Һ�е������ȫ��Ӧ���Һͱ�����ĺ�������ͬ���������ɵ��������Ҳ��ͬ�����Һͱ�����û�е���Ĵ�����ӣ����ŷ�Ӧ�Ľ��У���һ���ִ���Ҳ�����������ӣ�������п�۹�������������ɵ�������࣬����������ǿ�ᣬ�����ڴ˹������Բ���������������Բ��������˳������=��>�ס�
��2���ٷ�Ӧ�����������������һ���࣬������ֻ��п�����ᷴӦ�����ɵ�����һ������ݵ�ʧ�����غ�����ж����ĵ�п��ҲӦ��һ���࣬��=�ң���֧�Թ���п�۾�û��ʣ�࣬�����к�������ͭ��Һ����п�۷�Ӧ����п�ۣ����Ա��м����п�۱Ƚ϶࣬���Լ���п�۵�������СΪ��>��=�ҡ��ڱ��д�������ͭ��п�����䷴Ӧ����ͭ�������γ�ԭ��أ����Է�Ӧ��죬����ʱ����̣��������ŷ�Ӧ�Ľ�����Һ�еĴ�����ӵ���ƽ�������ķ�����У���Һ�е������ӵõ����ʵ��IJ��䣬�����Թ��������Ӳ�������Ũ�ȼ�С��Ӧ���ʼ�С����Ӧ����������ʱ��������Է�Ӧ�����ʱ��Ϊ��>��>������п�۹�����������Һ�е������ȫ��Ӧ���Һͱ�����ĺ�������ͬ���������ɵ��������Ҳ��ͬ�����Һͱ�����û�е���Ĵ�����ӣ����ŷ�Ӧ�Ľ��У���һ���ִ���Ҳ�����������ӣ�������п�۹�������������ɵ�������࣬����������ǿ�ᣬ�����ڴ˹������Բ���������������Բ��������˳������=��>�ס�

��ϰ��ϵ�д�
 ��Уͨ��֤��Ч��ҵϵ�д�
��Уͨ��֤��Ч��ҵϵ�д�
�����Ŀ