��Ŀ����
��12�֣���ͼ��ʵ������ȡ����������ʵ��װ�á���ش�
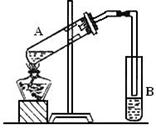
��1����M�Թ��м����Ƭ�������� ��
�Թ�N��ʢ�ŵ���Һ��_________________ ��
����Һ������Ϊ�к����ᣬ���ջӷ������Ҵ��� ��
�������� Һ���϶�������Һ���µ�ԭ���� ��
��2���ڴ��Թ�������һ���������Ҵ���Ũ���������
���Һʱ�������Լ���˳���� ��
��3��Ũ����������� ��
��4����Ӧ�������Թ�N�ڵ�Һ��ֳ����㣬��Ӧ���ɵ����������� �㣨��д���ϡ����¡�����������N�е�Һ��������Ҫ�õ��IJ����������ձ��� ��
��5��M�Թ��з�����Ӧ�Ļ�ѧ����ʽ ��
��6���������������ķ�Ӧ�ǿ��淴Ӧ����Ӧ�ﲻ����ȫ����������Ӧһ��ʱ���
�ʹﵽ�˸÷�Ӧ���ȣ�Ҳ���ﵽ��ѧƽ��״̬������������˵���Ҵ����������
����Ӧ�Ѵﵽ��ѧƽ��״̬��������������������(�����)��
�ٵ�λʱ�������1mol����������ͬʱ����1molˮ
�ڵ�λʱ�������1mol����������ͬʱ����1mol����
�۵�λʱ�������1mol�Ҵ���ͬʱ����1mol����

��1����M�Թ��м����Ƭ�������� ��
�Թ�N��ʢ�ŵ���Һ��_________________ ��
����Һ������Ϊ�к����ᣬ���ջӷ������Ҵ��� ��
�������� Һ���϶�������Һ���µ�ԭ���� ��
��2���ڴ��Թ�������һ���������Ҵ���Ũ���������
���Һʱ�������Լ���˳���� ��
��3��Ũ����������� ��
��4����Ӧ�������Թ�N�ڵ�Һ��ֳ����㣬��Ӧ���ɵ����������� �㣨��д���ϡ����¡�����������N�е�Һ��������Ҫ�õ��IJ����������ձ��� ��
��5��M�Թ��з�����Ӧ�Ļ�ѧ����ʽ ��
��6���������������ķ�Ӧ�ǿ��淴Ӧ����Ӧ�ﲻ����ȫ����������Ӧһ��ʱ���
�ʹﵽ�˸÷�Ӧ���ȣ�Ҳ���ﵽ��ѧƽ��״̬������������˵���Ҵ����������
����Ӧ�Ѵﵽ��ѧƽ��״̬��������������������(�����)��
�ٵ�λʱ�������1mol����������ͬʱ����1molˮ
�ڵ�λʱ�������1mol����������ͬʱ����1mol����
�۵�λʱ�������1mol�Ҵ���ͬʱ����1mol����
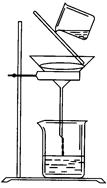
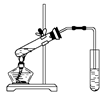
��1����ֹ���С�����̼������Һ�����������������ܽ�ȣ����ڷֲ㡣��ֹ������
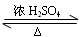
��2���ȼ����Ҵ����ڻ�������Ũ���ᣬ��ȴ���ڼ������ᡣ
��3����������ˮ����
��4���ϣ���Һ©����
��5��CH3COOH + C2H5OH CH3COOC2H5 + H2O
��6����

��2���ȼ����Ҵ����ڻ�������Ũ���ᣬ��ȴ���ڼ������ᡣ
��3����������ˮ����
��4���ϣ���Һ©����
��5��CH3COOH + C2H5OH CH3COOC2H5 + H2O
��6����
���������������Ʊ���

��1��������Ӧ��Ҫ���ȣ�Ϊ�˷�ֹҺ�����ʱ������������Ҫ�������Ƭ�Է�ֹ���С�������Ҵ����ӷ����������ɵ����������к���������Ҵ������뱥��̼����һ������������Ҵ����к����ᣬ��һ������Խ��������������ܽ�ȣ������ڷֲ�������������Ҵ���ˮ���ܣ����Բ���ֱ�Ӳ�����Һ�У��Է�ֹ������
��2��Ũ��������ˮ�ų��������ȣ���Ũ������ܶȴ���ˮ�ģ�Ϊ�˷�ֹ������Ҵ��Ļӷ���Ӧ���ȼ����Ҵ����ڻ�������Ũ���ᣬ��ȴ���ڼ������ᡣ
��3����Ϊ�ǿ��淴Ӧ������Ũ����������������⣬������ˮ�������ã�ʹ��Ӧ���������������������ķ����ƶ���
��4�������������ܶȱ�ˮ��С����������ˮ���������ϲ㣬ͨ����Һ©������ʵ�ַ��롣
��5����Ӧ����ʽΪCH3COOH + C2H5OH CH3COOC2H5 + H2O��
��6���٢��з�Ӧ���ʵķ�������ͬ�ģ����κ�����º�������ڷ�Ӧ���ʵķ������෴�ģ�����������֮������Ӧ�Ļ�ѧ������֮�ȣ���ȷ��

��1��������Ӧ��Ҫ���ȣ�Ϊ�˷�ֹҺ�����ʱ������������Ҫ�������Ƭ�Է�ֹ���С�������Ҵ����ӷ����������ɵ����������к���������Ҵ������뱥��̼����һ������������Ҵ����к����ᣬ��һ������Խ��������������ܽ�ȣ������ڷֲ�������������Ҵ���ˮ���ܣ����Բ���ֱ�Ӳ�����Һ�У��Է�ֹ������
��2��Ũ��������ˮ�ų��������ȣ���Ũ������ܶȴ���ˮ�ģ�Ϊ�˷�ֹ������Ҵ��Ļӷ���Ӧ���ȼ����Ҵ����ڻ�������Ũ���ᣬ��ȴ���ڼ������ᡣ
��3����Ϊ�ǿ��淴Ӧ������Ũ����������������⣬������ˮ�������ã�ʹ��Ӧ���������������������ķ����ƶ���
��4�������������ܶȱ�ˮ��С����������ˮ���������ϲ㣬ͨ����Һ©������ʵ�ַ��롣
��5����Ӧ����ʽΪCH3COOH + C2H5OH CH3COOC2H5 + H2O��
��6���٢��з�Ӧ���ʵķ�������ͬ�ģ����κ�����º�������ڷ�Ӧ���ʵķ������෴�ģ�����������֮������Ӧ�Ļ�ѧ������֮�ȣ���ȷ��

��ϰ��ϵ�д�
�����Ŀ
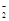 ��Al(OH)3
��Al(OH)3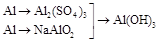
 ��g��NaOH���塣����������ƽ�������Ϸ��루
��g��NaOH���塣����������ƽ�������Ϸ��루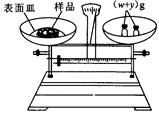
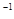 NaOH��Һ���ټ���������м������Һ�Լ��ȡ��������ǣ�__________________________������ˮ����м��ϴ�ɾ���������м������Ϊm1(g)��
NaOH��Һ���ټ���������м������Һ�Լ��ȡ��������ǣ�__________________________������ˮ����м��ϴ�ɾ���������м������Ϊm1(g)��