��Ŀ����
��7�֣��о�ijһ��ѧ��Ӧ��ʵ��װ��������ͼ��ʾ��A��F�������������е��������ʣ�Ũ���ᡢŨ���ᡢŨ��ˮ��ϡ���ᡢϡ���ᡢϡ��ˮ��ˮ��п����ͭƬ��ʳ�Ρ�������ء��Ȼ��ơ������ơ�������������������������ͭ��������������̼������������һ����̼���������Ȼ��⡢������������������ʵ������D�����ɺ�ɫ��Ϊ��ɫ����ˮ����ͭ��ĩ������ɫ����E�еõ���ɫ��Һ�۵�ȼ��þ��������ɫ��ζ��F��þ����������һ�ֹ������ʡ��������ʷ���ˮ�У�������ų�����������д̼�����ζ������ʹ��ʪ�ĺ�ɫʯ����ֽ������ͨ�������ش�

��1��д��A��F���������ƣ�A. ��B. �� E. ��
��2��д���йصĻ�ѧ����ʽ�� C��D ��
F��Mg�ķ�Ӧ������ˮ ��

��1��д��A��F���������ƣ�A. ��B. �� E. ��
��2��д���йصĻ�ѧ����ʽ�� C��D ��
F��Mg�ķ�Ӧ������ˮ ��
(1)д��A��F���������ƣ�A.Ũ��ˮ��B.�����ƣ�E.ˮ��������1�֣�
(2)д���йصĻ�ѧ����ʽ�� C��D��2NH3+3CuO��==3Cu+N2+3H2O����2�֣�
F��Mg�ķ�Ӧ������ˮ��Mg3N2+6H2O=3Mg(OH)2��+2NH3������2�֣�
(2)д���йصĻ�ѧ����ʽ�� C��D��2NH3+3CuO��==3Cu+N2+3H2O����2�֣�
F��Mg�ķ�Ӧ������ˮ��Mg3N2+6H2O=3Mg(OH)2��+2NH3������2�֣�
��

��ϰ��ϵ�д�
 Сѧ��ĩ���Ծ�ϵ�д�
Сѧ��ĩ���Ծ�ϵ�д�
�����Ŀ



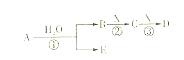
 H2S��������Һ������E��C�г���dz��ɫ���ǵ�����д��C�з�����Ӧ�Ļ�ѧ����ʽΪ
H2S��������Һ������E��C�г���dz��ɫ���ǵ�����д��C�з�����Ӧ�Ļ�ѧ����ʽΪ  �ʣ�����A��IΪ��������������֮������ϵ��ͼ��ʾ�����ַ�Ӧ�������û���г���������֪GΪ����Ԫ�صĹ�̬�����A��B��C��D��E��F���������о���ͬһ��Ԫ�ء�
�ʣ�����A��IΪ��������������֮������ϵ��ͼ��ʾ�����ַ�Ӧ�������û���г���������֪GΪ����Ԫ�صĹ�̬�����A��B��C��D��E��F���������о���ͬһ��Ԫ�ء�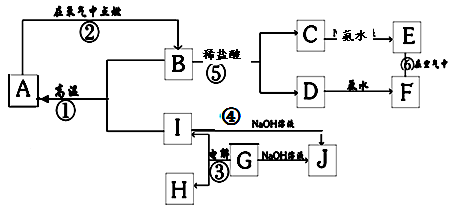
 ��ɫʯ����ֽ������X��F��YΪ���嵥�ʣ���YΪ��ɫ���壬FΪ��������Ҫ�ɷ�֮һ��GΪ���Σ�G����ɫ����M����Һ�пɷ�Ӧ����B��
��ɫʯ����ֽ������X��F��YΪ���嵥�ʣ���YΪ��ɫ���壬FΪ��������Ҫ�ɷ�֮һ��GΪ���Σ�G����ɫ����M����Һ�пɷ�Ӧ����B��

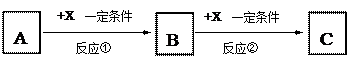
 ������ţ�
������ţ� �����ʣ�A�����嵥�ʣ���C��ˮ��Һ�еμ�AgNO3��Һ������������ϡHNO3�İ�ɫ������X������A��ȼ�ղ����ػ�ɫ���̡�
�����ʣ�A�����嵥�ʣ���C��ˮ��Һ�еμ�AgNO3��Һ������������ϡHNO3�İ�ɫ������X������A��ȼ�ղ����ػ�ɫ���̡�