��Ŀ����
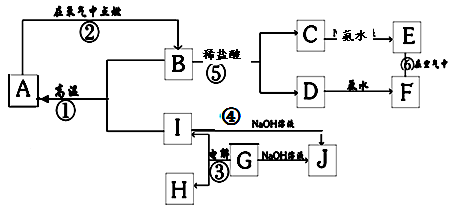
�� 12�� ��A~I�ֱ��ʾ��ѧ��ѧ�г�����һ���� �ʣ�����A��IΪ��������������֮������ϵ��ͼ��ʾ�����ַ�Ӧ�������û���г���������֪GΪ����Ԫ�صĹ�̬�����A��B��C��D��E��F���������о���ͬһ��Ԫ�ء�
�ʣ�����A��IΪ��������������֮������ϵ��ͼ��ʾ�����ַ�Ӧ�������û���г���������֪GΪ����Ԫ�صĹ�̬�����A��B��C��D��E��F���������о���ͬһ��Ԫ�ء�
����д���пհף�
��1��A��B��C��D��E��F����������������ͬһ��Ԫ����Ԫ�����ڱ��е�λ���� ��
��2������C��Һ�������ӵķ����ǣ�д�������������ۣ�
��
��3��д�����з�Ӧ�Ļ�ѧ����ʽ�����ӷ���ʽ��
��Ӧ�ٵĻ�ѧ����ʽ�� ��
��Ӧ�ܵ����ӷ���ʽ�� ��
��Ӧ�Ļ�ѧ����ʽ�� ��
��4���������仯�ĽǶȿ�����Ӧ�٢ڢ��У����ڡ�H��0�ķ�Ӧ�� ������ţ���
 �ʣ�����A��IΪ��������������֮������ϵ��ͼ��ʾ�����ַ�Ӧ�������û���г���������֪GΪ����Ԫ�صĹ�̬�����A��B��C��D��E��F���������о���ͬһ��Ԫ�ء�
�ʣ�����A��IΪ��������������֮������ϵ��ͼ��ʾ�����ַ�Ӧ�������û���г���������֪GΪ����Ԫ�صĹ�̬�����A��B��C��D��E��F���������о���ͬһ��Ԫ�ء�
����д���пհף�
��1��A��B��C��D��E��F����������������ͬһ��Ԫ����Ԫ�����ڱ��е�λ���� ��
��2������C��Һ�������ӵķ����ǣ�д�������������ۣ�
��
��3��д�����з�Ӧ�Ļ�ѧ����ʽ�����ӷ���ʽ��
��Ӧ�ٵĻ�ѧ����ʽ�� ��
��Ӧ�ܵ����ӷ���ʽ�� ��
��Ӧ�Ļ�ѧ����ʽ�� ��
��4���������仯�ĽǶȿ�����Ӧ�٢ڢ��У����ڡ�H��0�ķ�Ӧ�� ������ţ���
��12�֣�ÿ��2�֣���1����4���ڢ��壨2��ȡ������Һ���Թ��У��μ�KSCN��Һ����Һ��ɫ���μ���ˮ����Һ��ΪѪ��ɫ֤��C��Һ�к�Fe2+
(3)8Al+3Fe3O4 4Al2O3+9Fe
4Al2O3+9Fe
2Al+2OH- +6H2O =2[Al(OH)4]-+3H2��
4Fe(OH)2+O2+2H2O=4Fe(OH)3 (4) �٢�
(3)8Al+3Fe3O4
 4Al2O3+9Fe
4Al2O3+9Fe 2Al+2OH- +6H2O =2[Al(OH)4]-+3H2��
4Fe(OH)2+O2+2H2O=4Fe(OH)3 (4) �٢�
��

��ϰ��ϵ�д�
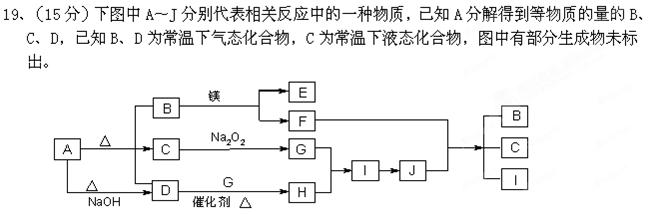
�����Ŀ

 B+þ �� E + F
B+þ �� E + F  ��
��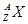 �������Ȼ���XCl2 2.22g����ˮ�Ƴ���Һ����2mol/L��
�������Ȼ���XCl2 2.22g����ˮ�Ƴ���Һ����2mol/L��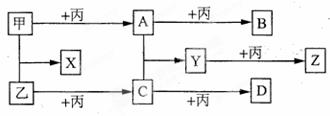
 , ������������ˮ���ﺬ�еĻ�ѧ��������_____________________.���Ȼ��ᄃ���е���λ����________
, ������������ˮ���ﺬ�еĻ�ѧ��������_____________________.���Ȼ��ᄃ���е���λ����________  ��ѧ����ʽ��______________.
��ѧ����ʽ��______________.