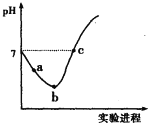
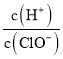��Ŀ����
����Ŀ������Ԫ��W��X��Y��Z��ԭ�������������ӣ��Ҿ�������20��W��X��Z������֮��Ϊ10��Y��ԭ�Ӱ뾶��ͬ��������Ԫ������С��W��Z�γɵĻ����ﳣ���¿��뱥��NaCl��Һ��Ӧ�����������峣�����и�ͺ��ӽ���������˵���������(����)
A.X�ĵ��������ཫ̫����ת��Ϊ���ܵij��ò���
B.YԪ�ؿ����γɶ��ֺ�����
C.XԪ������Ȼ����ֻ�л���̬û������̬
D.W��Z�γɵĻ������뱥��NaCl��Һ�ķ�Ӧ���������������ֻ�����Լ�
���𰸡�D
��������
�������и�ͺ��ӽ�������������Ȳ��W��Z�γɵĻ����ﳣ���¿��뱥��NaCl��Һ��Ӧ������Ȳ����W��Z�γɵĻ�������CaC2��W��Z�ֱ���C��Ca��W��X��Z������֮��Ϊ10����X������������A�壬X��SiԪ�أ�Y��ԭ�Ӱ뾶��ͬ��������Ԫ������С��Y��ClԪ�ء�
A.����SiΪ���õİ뵼����ϣ������ཫ̫����ת��Ϊ���ܵij��ò��ϣ���A��ȷ��
B.ClԪ�غ��ж��ֻ��ϼۣ������γɶ��ֺ����ᣬ������ᡢ���ᡢ������ȣ���B��ȷ��
C.SiΪ����Ԫ�أ�����Ȼ����ֻ�Ի���̬���ڣ�û������̬����C��ȷ��
D.̼������ˮ��Ӧ������Ȳ(C2H2)���壬C2H2�Ľṹʽ��![]() ���÷����м��м��Լ����зǼ��Լ�����D����
���÷����м��м��Լ����зǼ��Լ�����D����
��ѡD��

 ͬ��������ϰϵ�д�
ͬ��������ϰϵ�д� �ο�ͨ�γ̱�˼ά����������ѵ��ϵ�д�
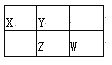
�ο�ͨ�γ̱�˼ά����������ѵ��ϵ�д�����Ŀ�����и��������У�����֮��ͨ��һ����Ӧ����ʵ����ͼ��ʾת�����ǣ�������
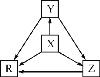
ѡ�� | X | Y | Z | R |
A | Al | AlCl3 | Al(OH)3 | NaAlO2 |
B | Na | Na2O | Na2O2 | NaOH |
C | H2S | S | SO2 | SO3 |
D | N2 | NH3 | NO | NO2 |
A.AB.BC.CD.D
����Ŀ��ijͬѧ���к͵ζ����ⶨij�ռ���Ʒ�Ĵ��ȡ�ʵ�鲽�����£�
��.���ƴ���Һ����������ƽ����5.0g�ռ���Ʒ�����ʲ������ᷴӦ�������Ƴ�1000mL��Һ��
��.�ζ���ȡ20.00mL�������Һ��0.10mol/L�������Һ���еζ����ﵽ�ζ��յ���ظ��˲������Ρ�
��.��¼�������£�
�ⶨ��� | ������Һ�������mL�� | ���������Һ�������mL�� | |
�ζ�ǰ���� | �ζ������ | ||
1 | 20.00 | 0.50 | 20.64 |
2 | 20.00 | 1.20 | 24.32 |
3 | 20.00 | 1.30 | 21.40 |
���������ʵ��ش��������⣺
��1�����ƴ���Һʱ�����ձ��Ͳ������⣬����Ҫ�õ�����Ҫ����������_____��
��2��ʢװ0.10mol/L�������ҺӦ��ʹ��____�ζ��ܣ�ѡ������ʽ��������ʽ�������ζ�ʱ�۾�Ӧע��۲�_____��
��3��������ۣ���ѡ����ƫ��������ƫ����������Ӱ������
��������ˮ��ϴ��ƿ���ⶨ���_____��
���ڵζ������в�����������Һ������ƿ�⣬�ⶨ���____��
�۶���ʱ���ζ�ǰ���ӣ��ζ������ӣ��ⶨ���_____��
��4�����㴿�ȣ��ռ���Ʒ�Ĵ�����_____(����һλС��)��
����Ŀ����1����0.3mol����̬����ȼ�������飨B2H6����������ȼ�գ����ɹ�̬�����������Һ̬ˮ���ų�649.5kJ�������÷�Ӧ���Ȼ�ѧ����ʽΪ______________������֪��H2O��g��=H2O��l������H2����44.0kJ/mol����11.2L����״������������ȫȼ��������̬ˮʱ�ų���������_____________kJ��
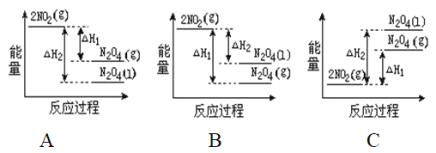
��2����֪��2NO2��g��![]() N2O4��g����H1 2NO2��g��
N2O4��g����H1 2NO2��g��![]() N2O4��l����H2
N2O4��l����H2
���������仯ʾ��ͼ�У���ȷ���ǣ�ѡ����ĸ��_____________��
��3�����ݸ�˹���ɿ��Զ�ijЩ����ͨ��ʵ��ֱ�Ӳⶨ�Ļ�ѧ��Ӧ���ʱ�������㡣
��֪��C��s��ʯī����O2��g����CO2��g�� ��H1����393.5 kJ��mol��1
2H2��g����O2��g����2H2O��l�� ��H2����571.6 kJ��mol��1
2C2H2��g����5O2��g����4CO2��g����2H2O��l�� ��H3����2 599 kJ��mol��1
���ݸ�˹���ɣ�����298 Kʱ��C��s��ʯī����H2��g������1 mol C2H2��g����Ӧ���ʱ䣨�г��ļ���ʽ����___________________________��
��4���״���һ�����͵���������ȼ�ϣ���ҵ�Ͽ�ͨ��CO��H2�������Ʊ��״����壨�ṹ��ʽΪCH3OH���� ��֪ijЩ��ѧ���ļ����������±���
��ѧ�� | C��C | C��H | H��H | C��O | C��O | H��O |
����/kJ��mol��1 | 348 | 413 | 436 | 358 | 1072 | 463 |
��֪CO�е�C��O֮��Ϊ�������ӣ���ҵ�Ʊ��״����Ȼ�ѧ����ʽΪ_________��
����Ŀ��Ϊ�˲ⶨʵ���ҳ��ڴ�ŵ�Na2SO3����Ĵ��ȣ�ȷ��ȡM g������Ʒ�����250 mL��Һ���������������ʵ�鷽����
����I��ȡ50.00 mL������Һ�����������������ữ��BaCl2��Һ������I��ϴ�ӡ���������������õ�����������Ϊm1 g
������ȡ50.00 mL������Һ����a mol/L ������KMnO4��Һ���еζ���
ʵ��������¼���������±���
�ζ����� ʵ������ | 1 | 2 | 3 | 4 |
������Һ���/mL | 50.00 | 50.00 | 50.00 | 50.00 |
�ζ��ܳ�����/mL | 0.00 | 0.20 | 0.10 | 0.15 |
�ζ���ĩ����/mL | 20.95 | 21.20 | 20.15 | 21.20 |
(1)����250 mL Na2SO3��Һʱ�������õ���ʵ�������У��ձ����������ιܡ�ҩ��_______��________��
(2)����IΪ______________��������Ϊ______________��
(3)�ڷ������еζ��յ���жϷ�����_______________________________��
(4)�ڷ������з��������ӷ�Ӧ����ʽΪ____________________________��
(5)���ݷ��������ṩ�����ݣ�����Na2SO3�Ĵ���Ϊ___________����д�ɷ�����ʽ��
(6)��������������ԭ�ζ������У����´���ҺNa2SO3Ũ�ȱ�С����_____������ţ���
a���ü�ʽ�ζ�����ȡ50mL����Һ����ʱ����ʼ���ӣ��ζ�����ʱ����
b���ü�ʽ�ζ�����ȡ50mL����Һ����ʱ��һ��ʼ�����ݣ��ζ�������û����
c����ʽ�ζ���������ˮ��ϴ��û��������KMnO4��Һ�����ϴ
d����ƿ������ˮ��ϴ��ֱ��װ50.00mL�Ĵ���Һ
e���ζ�����ʱ����ʼʱƽ�ӣ��ζ�����ʱ����