��Ŀ����
����Ŀ����һ���¶�ʱ��N2��H2��Ӧ�����������仯��������ͼ������a��ʾ��ʹ�ô���ʱ�������仯���ߣ�b��ʾʹ�ô���ʱ�������仯���ߡ�����������ȷ����

A. ״̬M��N����ʾ2molN(g)+6mol H(g)
B. �÷�Ӧ���Ȼ�ѧ����ʽΪ��N2+3H2![]() 2NH3 ��H=-92kJ��mol-l
2NH3 ��H=-92kJ��mol-l
C. ʹ�ô����������˷�Ӧ�����������������������˷�Ӧ�ų�������
D. ʹ�ô����������ܸı䷴Ӧ�ġ�H
���𰸡�D
��������A��״̬M��N��������ͬ��������Dz����ܱ�ʾ������ͬ�����ʣ��ʴ���B��д�Ȼ�ѧ��Ӧ����ʽ��Ҫ�������ʵ�״̬���ʴ���C�� �������ͻ�ܣ��ʱ���ʼ̬����̬�йأ�ʼ̬����̬���䣬��Ӧ�Ȳ��䣬�ʴ���D������ѡ��C�ķ���������ȷ��

 ��ѧ��ʦ����ϵ�д�
��ѧ��ʦ����ϵ�д�����Ŀ�����Ļ������ڹ�ҵ��Ӧ��ʮ�ֹ㷺��
��1����ҵ��������CO2��NH3��һ�������ºϳɣ����ȷ�Ӧ����ʽCO2(g)+2NH3(g)=CO(NH2)2(g)+H2O(g); H=____________��
��ѧ�� | ���ܣ�KJ��mol-1�� |
C��O | 728 |
C��N | 305 |
N��H | 389 |
O��H | 464 |
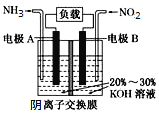
��2����ҵ�Ͼ���ʹ��NaClO��NH3��ȡ������������N2H4��Ϊ��Ԫ�����ˮ�еĵ��뷽ʽ�백���ơ�д�������ĵ���ʽ_________________�������������γɵ���ʽ�εĻ�ѧʽΪ__________________________________������Ҳ������Ϊȼ�ϵ�صĻ���ԭ��ʹ�ã�д�������ڼ��Ե������Һ�У������ĵ缫����ʽ_______________________________��
��3����֪�ϳɰ���Ӧ�ķ���ʽΪ N2+3H2![]() 2NH3����ƽ�ⳣ�� K ����ֵ���¶ȵĹ�ϵ������
2NH3����ƽ�ⳣ�� K ����ֵ���¶ȵĹ�ϵ������
�¶��� | 200 | 300 | 400 |
ƽ�ⳣ��K | 1 | 0.86 | 0.5 |
�����ϱ����ݿ�֪�÷�ӦH____0 (����>������<��)��
��400 ��ʱ�����ijʱ�� c(N2)= 5 mol��L-1��c(H2)= 3 mol��L-1��c(NH3)= 2 mol��L-1����ʱ�̸÷�Ӧ�� v ��____v ��(����>����=������<��)���÷�Ӧ�ﵽƽ��ʱ��ƽ��������N2���������Ϊ____________
A��50% B��60% C��70% D��������
��4��д�����к������������ض������µ�ˮ�ⷴӦ���縺��Cl��N�������е�C��+4�ۣ�
��NCl3___________________________________________________________________________________________________________
��NF3____________________________________________________________________________________________________________