��Ŀ����
3����֪X��Y��Z��W�Ƕ����������ַǽ���Ԫ�أ����ǵ�ԭ��������������XԪ��ԭ�Ӽ۵����Ų�ʽΪns1��YԪ���ǿ����к�������Ԫ�أ�Z ��Wͬ���壬����Wԭ�ӵĵ����Ų�ʽ�У�p�����ֻ��1��δ�ɶԵ��ӣ���1��д��Ԫ�ط��ţ�XH��YO��ZF��WCl��
��2����Ԫ��X��Ԫ��Y�γɵĻ��������ʽ��
 ������������ˮ������Ϊ����ˮ���Ӽ������γ����������WԪ�ص��⻯�ﷴӦ�γɵĻ������������Ӿ��壻
������������ˮ������Ϊ����ˮ���Ӽ������γ����������WԪ�ص��⻯�ﷴӦ�γɵĻ������������Ӿ��壻��3�������ַǽ���Ԫ���У��縺������Ԫ����F�����Ļ�̬ԭ�ӵĵ����Ų�ʽ��1s22s22p5��
���� ��1��X��Y��Z��W�Ƕ����������ַǽ���Ԫ�أ����ǵ�ԭ��������������XԪ��ԭ�Ӽ۵����Ų�ʽΪns1��X���ڵڢ�A�壬����X����Ԫ�أ�YԪ���ǿ����к�������Ԫ�أ�����Y�ǵ�Ԫ�أ�Z ��Wͬ���壬����Wԭ�ӵĵ����Ų�ʽ�У�p�����ֻ��1��δ�ɶԵ��ӣ�����W����Ԫ�أ�Z�Ƿ�Ԫ�أ�
��2��Ԫ��X��Ԫ��Y�γɰ��������ݵ�ԭ�Ӻ�һ����ԭ��֮�����һ�����õ��Ӷԣ�һ����ԭ�����γ�3�����õ��Ӷ���д����ʽ��
����������ˮ���Ӽ����γ�������Ȼ���������Ӿ��壻
��3��ͬ����������ҵ縺������ͬ�������϶��µ縺�Լ�С������FԪ��Ϊ9��Ԫ�أ��ж�Ԫ��ԭ�ӵĺ��������Ϊ9���ٸ��ݺ�������Ų�������д���ݴ˽��
��� �⣺��1��X��Y��Z��W�Ƕ����������ַǽ���Ԫ�أ����ǵ�ԭ��������������XԪ��ԭ�Ӽ۵����Ų�ʽΪns1��X���ڵڢ�A�壬����X����Ԫ�أ�YԪ���ǿ����к�������Ԫ�أ�����Y�ǵ�Ԫ�أ�Z ��Wͬ���壬����Wԭ�ӵĵ����Ų�ʽ�У�p�����ֻ��1��δ�ɶԵ��ӣ�����W����Ԫ�أ�Z�Ƿ�Ԫ�أ�
�ʴ�Ϊ��H��O��F��Cl��
��2����Ԫ��X��Ԫ��Y�γɵij����������ǰ�������ԭ�Ӻ�һ����ԭ��֮�����һ�����õ��Ӷԣ�һ����ԭ�����γ�3�����õ��Ӷԣ����������ʽΪ ���������ӺͰ������ӡ�����������ˮ���Ӽ����γ���������°�������Һ����Ҳ��������ˮ���Ȼ����笠����Ӻ�������֮��������Ӽ��������Ȼ���γɵľ��������Ӿ��壬
���������ӺͰ������ӡ�����������ˮ���Ӽ����γ���������°�������Һ����Ҳ��������ˮ���Ȼ����笠����Ӻ�������֮��������Ӽ��������Ȼ���γɵľ��������Ӿ��壬
�ʴ�Ϊ�� ���⣻���ӣ�
���⣻���ӣ�
��3�������ַǽ���Ԫ��ΪH��O��F��Cl��O��F����ͬһ���ڣ�ͬ����������ҵ縺�����ʵ縺��O��F��F��Cl����ͬһ���壬ͬ�������϶��µ縺�Խ��ͣ��ʵ縺��Cl��F��H����Ԫ�����ڱ������Ͻǣ�O��F��Cl�縺�Զ�����H����FԪ�صĵ縺�����FԪ��Ϊ9��Ԫ�أ�ԭ�Ӻ�����9�����ӣ����Ժ�������Ų�ʽΪ��1s22s22p5��
�ʴ�Ϊ��F��1s22s22p5��
���� ���⿼����Ԫ�ص��ƶϡ�����ʽ����д���縺�ԵıȽϡ���̬ԭ�ӵĺ�������Ų�ʽ��д��֪ʶ�㣬��Ŀ�Ѷ��еȣ�����㶼�Ǹ߿����ȵ㣬Ӧע�ػ���֪ʶ�Ļ��ۣ�ע�����Ԫ���������е縺�Եĵݱ���ɣ�

| A�� | 120�� | B�� | 117.6�� | C�� | 116�� | D�� | 121�� |
| A�� | ��Ȼ����Һ��ʯ��������������Ҫ�ɷ־�Ϊ���� | |
| B�� | ú�к��б����ױ������ֻ���ԭ�ϣ���ͨ��ú�ĸ����� | |
| C�� | ʯ����Ҫ�Ǹ�����������������ϩ����ɵĻ���� | |
| D�� | ��ϩ������ϩ������ͨ���ۺϷ�Ӧ�õ��߷��Ӳ��� |
�����ӻ����ﺬ���Ӽ���Ҳ���ܺ����Լ���Ǽ��Լ�
�ڹ��ۻ����ﺬ���ۼ���Ҳ���ܺ����Ӽ�
�ۺ�����Ԫ�صĻ����ﲻһ�������ӻ�����
���ɷǽ���Ԫ����ɵĻ�����һ���ǹ��ۻ�����
���ɷ�����ɵ�������һ�����ڹ��ۼ�
������״̬�ܵ���Ļ����������ӻ����
| A�� | �٢ۢ� | B�� | �ڢܢ� | C�� | �ڢۢ� | D�� | �٢ۢ� |
| A�� | CH3F�ĵ���ʽ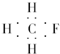 | B�� | Na2S�ĵ���ʽ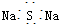 | C�� | �������ĵ���ʽ 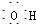 | D�� | ������ĵ���ʽ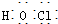 |
| A�� | ͭ��Ũ���ᷴӦ��Cu+4H++2NO3-=Cu2++2NO2��+2H2O | |
| B�� | ̼�������Һ��������NaOH��Һ��Ϻ���ȣ�NH4++OH-$\frac{\underline{\;\;��\;\;}}{\;}$NH3��+H2O | |
| C�� | ������ͭ�������2H++Cu��OH��2=Cu2++2H2O | |
| D�� | ������������ϡ���3Fe2++4H++NO3-=3Fe3++NO��+2H2O |
| A�� | H2O��Al2Cl6 | B�� | CO2��SO42- | ||
| C�� | PCl5��[Co��NH3��4Cl2]Cl | D�� | NH4Cl��[Cu��NH3��4]SO4 |
| A�� | ����������Ȼ�̼��Һ����ֱ�����ͺ��ѻ����� | |
| B�� | ��NaOH��AgNO3������Һ������֤����������Ԫ�صĴ��� | |
| C�� | ��ͭ˿�ھƾ��ƻ����ϼ��Ⱥ�����������ˮ�Ҵ��У�ͭ˿�ָ���ԭ���ĺ�ɫ | |
| D�� | ��������Һ�зֱ����ŨHNO3��10%NaOH��Һ��0.1%����ͪ��Һ��������ɫ���� |
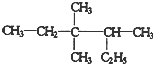 3��3��4-��������
3��3��4-�������� ��
�� ��
�� ���������ʽΪC10H16��������һ��ȡ������2�֣�
���������ʽΪC10H16��������һ��ȡ������2�֣� ��
��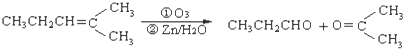 ������Ӧ�������ƶ���������̼̼˫����λ�ã�ij��A�ķ���ʽΪC6H10����������ת������
������Ӧ�������ƶ���������̼̼˫����λ�ã�ij��A�ķ���ʽΪC6H10����������ת������ 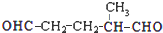 ������A�Ľṹ�ɱ�ʾΪ
������A�Ľṹ�ɱ�ʾΪ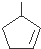 ��
��