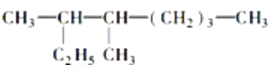��Ŀ����
����Ŀ�������£��������Һ�е����й�ϵʽ����ȷ����
A. ��֪A-+H2B(����)==HA+HB-������H+��������HB-<A-<B2-
B. ��0.1mol��L-1�İ�ˮ�еμ�0.1 mol��L-1���ᣬǡ����ȫ�к�ʱ�� c(NH4+)+c(NH3 H2O)=0.05 mol��L-1
C. ��c mol��L-1�Ĵ�����Һ��0.02 mol��L-1NaOH��Һ�������Ϻ���Һǡ�ó����ԣ��ú�c�Ĵ���ʽ��ʾCH3COOH�ĵ��볣��ka=![]()
D. ��0.2 mol��L-1������0.1 mol��L-1��KAlO2��Һ�������ϣ���Һ������Ũ���ɴ�С��˳��c(Cl-)> c(K+)> c(Al3+)> c(H+)> c(OH-)
���𰸡�A
��������A�����ݷ�Ӧ����ʽ��A�����H��������ǿ����H2B����HA��HB�������H�������������B2�������˳����A��>B2��>HB������A˵������B��ǡ����ȫ�кͣ���Ϊ����Ͱ�ˮ��Ũ����ȣ�������ĵ���������Ϊ�백ˮ�������ȣ����������غ㣬c(NH4��)��c(NH3��H2O)=0.1/2mol��L��1=0.05mol��L��1����B˵����ȷ��C�����ݵ���غ㣬c(CH3COO��)��c(OH��)=c(H��)��c(Na��)����Ϊ��Һ�����ԣ������c(OH��)=c(H��)=10��7mol��L��1������c(CH3COO��)=c(Na��)=0.02/2mol��L��1����ʱ��Һ��c(CH3COOH)=(c��0.02)/2mol��L��1�����ݵ��볣���Ķ��壬Ka=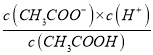 =
=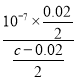 =
=![]() ����C˵����ȷ��D������������Ϊ1L�������ķ�Ӧ��HCl��KAlO2��H2O=Al(OH)3����KCl������Ͷ������KAlO2ȫ���μӷ�Ӧ�������������ʱʣ����������ʵ���Ϊ(0.2��0.1)mol=0.1mol������Al(OH)3��KCl�����ʵ���Ϊ0.1mol�����ŷ�����Al(OH)3��3HCl=AlCl3��3H2O������������㣬Al(OH)3����������AlCl3�����ʵ���Ϊ0.1/3mol��AlCl3����ǿ�������Σ���Һ�����ԣ��������Ũ�ȴ�С˳����c(Cl��)>c(K��)>c(Al3��)>c(H��)>c(OH��)����D˵����ȷ��
����C˵����ȷ��D������������Ϊ1L�������ķ�Ӧ��HCl��KAlO2��H2O=Al(OH)3����KCl������Ͷ������KAlO2ȫ���μӷ�Ӧ�������������ʱʣ����������ʵ���Ϊ(0.2��0.1)mol=0.1mol������Al(OH)3��KCl�����ʵ���Ϊ0.1mol�����ŷ�����Al(OH)3��3HCl=AlCl3��3H2O������������㣬Al(OH)3����������AlCl3�����ʵ���Ϊ0.1/3mol��AlCl3����ǿ�������Σ���Һ�����ԣ��������Ũ�ȴ�С˳����c(Cl��)>c(K��)>c(Al3��)>c(H��)>c(OH��)����D˵����ȷ��

����Ŀ��Ԫ��A�ĸ����������������£�
I1 | I2 | I3 | I4 | I5 | I6 | |
I/(kJ��mol-1) | 568 | 1517 | 9745 | 10978 | 13931 | 17978 |
��Ԫ��A������̬�ǣ� ��
A.+1B.+2C.+3D.+4
����Ŀ��ʵ������ȡ���ᶡ����ʵ��װ����������ͼ��ʾ����װ�ù�ѡ�á����й����ʵ���������
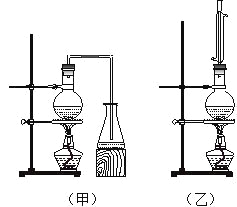
���±���
���� | 1-���� | ���ᶡ�� | |
�۵�(��) | 16.6 | ��89.5 | ��73.5 |
�е�(��) | 117.9 | 117 | 126.3 |
�ܶ�(g/cm3) | 1.05 | 0.81 | 0.88 |
ˮ���� | ���� | ����(9g/100gˮ) | �� |
��1����ȡ���ᶡ����װ��Ӧѡ��_______(����������������)����ѡ��һ��װ�õ������� ��
��2����ʵ���������г������������ᶡ���⣬���������ɵ��л��������У�д���ṹ��ʽ���� �� ��
��3��������Ӧ��һ�����淴Ӧ��Ϊ���1-�����������ʣ��ɲ�ȡ�Ĵ�ʩ�� ��
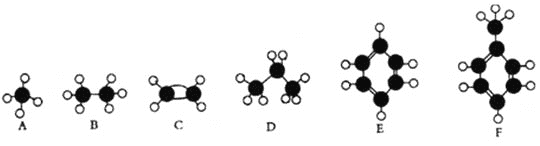
��4�����Ʊ����ᶡ�����õĻ�����з��롢�ᴿ���ᶡ��ʱ����Ҫ�����ಽ����������ͼʾ�IJ����У��϶���Ҫ�Ļ�ѧ������___________��ѡ��𰸱�ţ���
��5���л���ķ�������У�������Ҫʹ�÷�Һ©����������ʹ�÷�Һ©��ǰ���� ��ijͬѧ�ڽ��з�Һ����ʱ��������Һ�����������������ԭ�����Һ©�����������⣬������ ��