��Ŀ����
ʵ���ҿ��ø��﴿���İ���ԭ����ͭ����ȡ��������������װ��ͼ�����ּг�װ����ȥ���ش��й����⣺
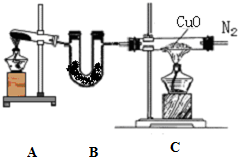
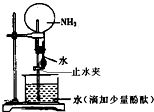
��1��װ��B��ʢ�ŵ�ҩƷ��______��
��2��д��װ��A�з�Ӧ�Ļ�ѧ����ʽ______��
��3��д��װ��C�з�Ӧ�Ļ�ѧ����ʽ______���÷�Ӧ�е���������______��
��4���ռ����ĵ����г��˺�������ˮ�����⣬�����ܺ�������һ�����壬ȷ���Ƿ��и������ʵ�鷽����______��ʵ�����ռ������ķ�����______��

��1��װ��B��ʢ�ŵ�ҩƷ��______��
��2��д��װ��A�з�Ӧ�Ļ�ѧ����ʽ______��
��3��д��װ��C�з�Ӧ�Ļ�ѧ����ʽ______���÷�Ӧ�е���������______��
��4���ռ����ĵ����г��˺�������ˮ�����⣬�����ܺ�������һ�����壬ȷ���Ƿ��и������ʵ�鷽����______��ʵ�����ռ������ķ�����______��
��1���Ʊ��İ����к���ˮ�����������Ǽ������壬ѡ�����������ʯ�ҳ�ȥˮ������
�ʴ�Ϊ����ʯ�ң�
��2��ʵ���������������ƺ��Ȼ�粒�����ȷ�Ӧ���ɣ���Ӧ�Ļ�ѧ����ʽΪ��Ca��OH��2+2NH4Cl
CaCl2+2NH3��+2H2O��
�ʴ�Ϊ��Ca��OH��2+2NH4Cl
CaCl2+2NH3��+2H2O��
��3������������ͭ����Ϊ����������ͭ����ԭΪͭ����Ӧ�Ļ�ѧ����ʽΪ��2NH3+3CuO
3Cu+N2��+3H2O��
�ʴ�Ϊ��2NH3+3CuO
3Cu+N2��+3H2O��
��4���ռ����ĵ����г��˺�������ˮ�����⣬�����ܺ�������һ�����就����ȷ���Ƿ��и������ʵ�鷽��������ʪ���ʯ����ֽ����������ڣ���ʪ��ĺ�ɫʯ����ֽ���������ܿڣ�����ֽ����ɫ��֤�����и����壬������������ˮ�ıȿ�����������壬ѡ����ˮ���������ռ���
�ʴ�Ϊ����ʪ��ĺ�ɫʯ����ֽ���������ܿڣ�����ֽ����ɫ��֤�����и����壬��ˮ������
�ʴ�Ϊ����ʯ�ң�
��2��ʵ���������������ƺ��Ȼ�粒�����ȷ�Ӧ���ɣ���Ӧ�Ļ�ѧ����ʽΪ��Ca��OH��2+2NH4Cl
| ||
�ʴ�Ϊ��Ca��OH��2+2NH4Cl
| ||
��3������������ͭ����Ϊ����������ͭ����ԭΪͭ����Ӧ�Ļ�ѧ����ʽΪ��2NH3+3CuO
| ||
�ʴ�Ϊ��2NH3+3CuO
| ||
��4���ռ����ĵ����г��˺�������ˮ�����⣬�����ܺ�������һ�����就����ȷ���Ƿ��и������ʵ�鷽��������ʪ���ʯ����ֽ����������ڣ���ʪ��ĺ�ɫʯ����ֽ���������ܿڣ�����ֽ����ɫ��֤�����и����壬������������ˮ�ıȿ�����������壬ѡ����ˮ���������ռ���
�ʴ�Ϊ����ʪ��ĺ�ɫʯ����ֽ���������ܿڣ�����ֽ����ɫ��֤�����и����壬��ˮ������

��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ