��Ŀ����
2��ʯ�ͻ�������Ҫԭ��CxHy���Ժϳɺܶ��л������������CxHy�ϳ�����E��J ������ͼ��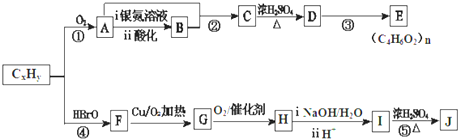
��֪�������з�Ӧ��R��R���������

��J�ķ���ʽΪC4H4O4����һ�ֻ�״�����
��1����CxHy��ͬϵ���У�����̼ԭ��һ����ƽ����̼ԭ�������ķ��ӵ�����2��3-����-2-��ϩ��
��2��H�ķ���ʽ��C2H3O2Br��
��3������˵����ȷ����be��
a��CxHy�ͱ�����ʹ��ˮ��ɫ��ԭ����ͬ
b����Ӧ�ںͷ�Ӧ�ܵķ�Ӧ���;�Ϊ�ӳɷ�Ӧ
c��C����Na��NaOH��NaHCO3��Ӧ
d��E��һ��ˮ���Ժܺõĸ߷��ӻ�����
e��J�����Ի���Ի����о���ˮ��
��4��K��J��ͬ���칹�壬��1mol K��������NaHCO3��Һ��Ӧ�ɷų�2mol CO2���壬��д��һ�ַ�������K�Ľṹ��ʽCH2=C��COOH��2��HOOCCH=CHCOOH
��5��д����Ӧ�ݵĻ�ѧ����ʽ
 ��
����6��D�ж���ͬ���칹�壬��D������ͬ�����ŵĻ���4�֣�������˳���칹����
���к˴Ź���������3�����շ壬���ܷ���������Ӧ�Ľṹ��ʽ��HCOOC��CH3��=CH2��
���� �������и�����ת����ϵ��J�ķ���ʽΪC4H4O4����һ�ֻ�״���������I��Ũ���������¼��ȵõ������Կ�����֪JΪ��������JΪ �������йط�Ӧ����������֪IΪHOCH2COOH��HΪBrCH2COOH��GΪBrCH2CHO��FΪBrCH2CH2OH��CxHy��HBrO�ӳɵ�F������CxHyΪCH2=CH2��������AΪCH3CHO��CH3CHO��������BΪCH3COOH��A��B������Ϣ�еļӳɷ�Ӧ��CΪCH3COOCH��OH��CH3��C��Ũ������������ˮ��DΪCH3COOCH=CH2��D�����Ӿ۷�Ӧ�õ�E���ݴ˴��⣮
�������йط�Ӧ����������֪IΪHOCH2COOH��HΪBrCH2COOH��GΪBrCH2CHO��FΪBrCH2CH2OH��CxHy��HBrO�ӳɵ�F������CxHyΪCH2=CH2��������AΪCH3CHO��CH3CHO��������BΪCH3COOH��A��B������Ϣ�еļӳɷ�Ӧ��CΪCH3COOCH��OH��CH3��C��Ũ������������ˮ��DΪCH3COOCH=CH2��D�����Ӿ۷�Ӧ�õ�E���ݴ˴��⣮
��� �⣺�������и�����ת����ϵ��J�ķ���ʽΪC4H4O4����һ�ֻ�״���������I��Ũ���������¼��ȵõ������Կ�����֪JΪ��������JΪ �������йط�Ӧ����������֪IΪHOCH2COOH��HΪBrCH2COOH��GΪBrCH2CHO��FΪBrCH2CH2OH��CxHy��HBrO�ӳɵ�F������CxHyΪCH2=CH2��������AΪCH3CHO��CH3CHO��������BΪCH3COOH��A��B������Ϣ�еļӳɷ�Ӧ��CΪCH3COOCH��OH��CH3��C��Ũ������������ˮ��DΪCH3COOCH=CH2��D�����Ӿ۷�Ӧ�õ�E��
�������йط�Ӧ����������֪IΪHOCH2COOH��HΪBrCH2COOH��GΪBrCH2CHO��FΪBrCH2CH2OH��CxHy��HBrO�ӳɵ�F������CxHyΪCH2=CH2��������AΪCH3CHO��CH3CHO��������BΪCH3COOH��A��B������Ϣ�еļӳɷ�Ӧ��CΪCH3COOCH��OH��CH3��C��Ũ������������ˮ��DΪCH3COOCH=CH2��D�����Ӿ۷�Ӧ�õ�E��
��1����C2H4��ͬϵ���У�����̼ԭ��һ����ƽ����̼ԭ�������ķ����ǽ���ϩ�е���ԭ�Ӷ�����̼�Ľṹ��C6H12����������2��3-����-2-��ϩ��
�ʴ�Ϊ��2��3-����-2-��ϩ�� ������������
��2��HΪBrCH2COOH��H�ķ���ʽ��C2H3O2Br��
�ʴ�Ϊ��C2H3O2Br��
��3��a��CxHy�ͱ�����ʹ��ˮ��ɫ��ԭ������ͬ��ǰ���Ǽӳɣ���������ȡ����a����
b����Ӧ�ںͷ�Ӧ�ܵķ�Ӧ���;�Ϊ�ӳɷ�Ӧ����b��ȷ��
c��C�����������ǻ������������������Ʒ�Ӧ���ǻ������Ʒ�Ӧ���������ǻ���������NaHCO3��Ӧ����c����
d��E������������ˮ���Խϲ��d����
e��J���������������Ի���Ի����о���ˮ�⣬��e��ȷ��
�ʴ�Ϊ��be��
��4��JΪ ��K��J��ͬ���칹�壬��1 mol K��������NaHCO3��Һ��Ӧ�ɷų�2mol CO2���壬˵��K���������Ȼ������Է�������K�Ľṹ��ʽΪCH2=C��COOH��2����HOOCCH=CHCOOH��
��K��J��ͬ���칹�壬��1 mol K��������NaHCO3��Һ��Ӧ�ɷų�2mol CO2���壬˵��K���������Ȼ������Է�������K�Ľṹ��ʽΪCH2=C��COOH��2����HOOCCH=CHCOOH��
�ʴ�Ϊ��CH2=C��COOH��2����HOOCCH=CHCOOH��
��5����Ӧ�ݵĻ�ѧ����ʽΪ ��
��
�ʴ�Ϊ�� ��
��
��6��DΪCH3COOCH=CH2��D�ж���ͬ���칹�壬��D������ͬ�����ŵĻ���CH2=CHCOOCH3��HCOOCH=CHCH3��HCOOCH2CH=CH2��HCOOC��CH3��=CH2����4�֣���˳���칹�壩�����к˴Ź���������3�����շ壬���ܷ���������Ӧ�Ľṹ��ʽΪHCOOC��CH3��=CH2��
�ʴ�Ϊ��4��HCOOC��CH3��=CH2��
���� ���⿼���л�����ƶ���ϳɣ���ϸ������Ϣ��������úϳ�·�������ʹ����ż�̼���ı仯�ƶϣ���Ŀ�Ѷ��еȣ�ע���л�����֪ʶ��������ã�

| ��� | ������ | ��ԭ�� | ������Ӧ�� | �������� | ��ԭ���� |
| �� | Cl2 | FeI2 | I2 | ||
| �� | KClO3 | Ũ���� | Cl2 | ||
| �� | KMnO4 | H2O2 | O2 | Mn2+ |
| A�� | ���Т���ķ�Ӧ����������һ����I2 | |
| B�� | ������ǿ���ıȽϣ�KClO3��Cl2��Fe3+��I2 | |
| C�� | ���Т���ÿ����1molCl2��ת��1mol���� | |
| D�� | ���Т���������Ӧ�����ΪŨ���� |
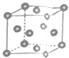
| A�� | ֻ�н�����ԭ�Ӳ������ֶѻ���ʽ | B�� | ���ֶѻ���ʽ�У���λ��Ϊ8 | ||
| C�� | ���ǽ��������һ�����ܶѻ���ʽ | D�� | ���ֶѻ���ʽ�Ŀռ������ʽϵ� |
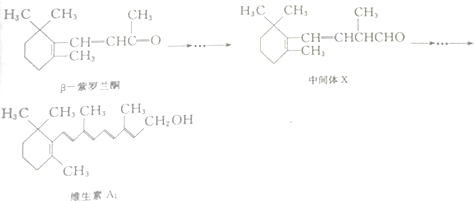
����˵����ȷ���ǣ�������
| A�� | ��-������ͪ�ں˴Ź�������ͼ����7��� | |
| B�� | ��-������ͪ���м���X��Ϊͬ���칹�� | |
| C�� | 1mol�м���X�������2mol H2�����ӳɷ�Ӧ | |
| D�� | ά����A1�ܷ���ȡ����Ӧ���ӳɷ�Ӧ��������Ӧ |

| A�� | �������ռӦ | B�� | ��˿��������Ӧ | C�� | �����ƺ�ˮ��Ӧ | D�� | ̼��ƷֽⷴӦ |
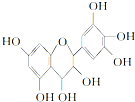
ԭ�����ؾ���������ԣ��翹���������ɻ���������ȣ��ɷ�ֹ������֬�����������ɻ��IJ����������������ȶ��ּ������й�ԭ�����ص�����˵������ȷ���ǣ�������
| A�� | �����ʼȿɿ������࣬Ҳ�ɿ������� | |
| B�� | 1 mol�����ʿ���4 mol Br2��Ӧ | |
| C�� | 1 mol�����ʿ���7 mol NaOH��Ӧ | |
| D�� | 1 mol�����ʿ���7 mol Na��Ӧ |
�� ���� | ��A | ��A | ��A | ��A | ��A | ��A | ��A | 0 |
| �� | �� | �� | �� | |||||
| �� | �� | �� | �� | �� | �� |

��2���ؿ��к�������Ԫ����O��
��3������ܵĵ����ڼ��������·�Ӧ����һ�ֵ���ɫ���壬�ù���ĵ���ʽΪ
 ��
����4����ЩԪ���е�����������Ӧ��ˮ�����У�������ǿ����HClO4��������ǿ����NaOH�������Ե�����������Al��OH��3������������ʻ�ѧʽ��
��5��д���ݵĵ���������������Һ��Ӧ�����ӷ���ʽ��2Al+2NaOH+2H2O=2NaAlO2+3H2����
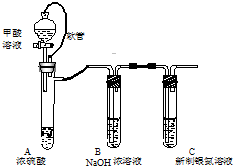 ������Һ�����ڼ��CO���壬ʵ�����о���װ����ͼ��
������Һ�����ڼ��CO���壬ʵ�����о���װ����ͼ�� ��
��