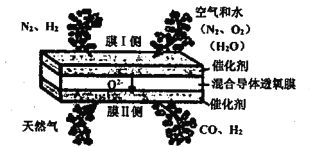
��Ŀ����
����Ŀ���Ի���������(��Ҫ�ɷ�ΪFe2O3��������FeS��SiO2��Cu��Au��Ag��)Ϊԭ���Ʊ���������(Fe2O3)������E������H���ؽ�������������������������ͼ��ʾ��

�밴Ҫ��ش��������⣺
(1)�������̵��м����(NH4)2Fe6(SO4)4(OH)12��Fe�Ļ��ϼ�Ϊ__________________��
Fe�����ڱ��е�λ��Ϊ��________���ڵڢ��塣
(2)�������ܽ�ǰ����������������¶�����Ŀ����________________________�����̢��ܽ����������Լ������ʵĻ�ѧʽΪ__________________��
��Ӧ�ڵĻ�ѧ����ʽ��______________________________________________________��
(3)��Ӧ�۵�Ŀ����____________________________________��
����Z�ĵ���ʽ��__________________��
д����Ӧ�ܵ����ӷ���ʽ��______________________________________________________��
(4)�����յ�G��������ˮ��������Һ���������ӵ�Ũ���ɴ�С������______________��
���𰸡�+3 �� ��������ܽ����ʺ��ܳ��� H2SO4 SiO2+2NaOH=Na2SiO3+H2O ��+2��������Ϊ+3���� ![]() 3NH3��H2O+Fe3+=Fe(OH)3��+3NH4+ c(NH4+)>c(SO42-)>c(H+)>c(OH-)
3NH3��H2O+Fe3+=Fe(OH)3��+3NH4+ c(NH4+)>c(SO42-)>c(H+)>c(OH-)
��������
��������ͼ������������(��Ҫ�ɷ�ΪFe2O3��������FeS��SiO2��Cu��Au��Ag��)�������ܽ⣬���ɵ���ҺA����Fe3+��Fe2+�������к���SiO2��������Cu��Au��Ag�ȣ����������������ܽ⣬�ɻ����ؽ��������Cu��Au��Ag�ȣ�ͬʱ�ù�������������ҺA�е�Fe2+�õ���ҺB��������Ϊ��������ͨ�백��������Ӧ�õ�����E����ˮ�ܽ����ͨ�백����������ת��Ϊ���������������������ȷֽ�õ����죬�ݴ˷�������
��1��(NH4)2Fe6(SO4)4(OH)12��NH4+��SO42-��OH-����ֱ���+1�ۡ�-2�ۡ�-1�ۣ�����Ԫ�ػ��ϼ�Ϊx�����ݻ�����Ļ��ϼ۴�����Ϊ0������+1��2 + 6x +(-2)��4 + (-1)��12 = +3��FeΪ26��Ԫ�أ������ڱ��е�λ��Ϊ�������ڵڢ��壬
�ʴ�Ϊ��+3���ģ�
��2���ܽ�ǰ����������������¶�����Ŀ������������ܽ����ʺ��ܳ��ʣ���������˼ά������Ԫ���غ��֪��Ϊ���Ʊ�����E����������������ӣ����Թ��̢��ܽ����������Լ������ʵĻ�ѧʽΪH2SO4����������������֪����Ӧ��Ϊ���������ܽ��������Ĺ��̣��仯ѧ����ʽΪ��SiO2+2NaOH=Na2SiO3+H2O��
�ʴ�Ϊ����������ܽ����ʺ��ܳ��ʣ�H2SO4��SiO2+2NaOH=Na2SiO3+H2O��
��3����ҺA����Fe3+��Fe2+��Ϊ���к����Ʊ��������轫������������Ϊ�����ӣ����Է�Ӧ�۵�Ŀ���ǽ�+2��������Ϊ+3����������ZΪ�����������ʽΪ��![]() ����Ӧ��Ϊ������ת��Ϊ�������������Ĺ��̣������ӷ���ʽΪ��3NH3��H2O+Fe3+=Fe(OH)3��+3NH4+��
����Ӧ��Ϊ������ת��Ϊ�������������Ĺ��̣������ӷ���ʽΪ��3NH3��H2O+Fe3+=Fe(OH)3��+3NH4+��
�ʴ�Ϊ����+2��������Ϊ+3������![]() ��3NH3��H2O+Fe3+=Fe(OH)3��+3NH4+��
��3NH3��H2O+Fe3+=Fe(OH)3��+3NH4+��
��4�����յ�G��������ˮ�õ�����ҺΪ(NH4)2SO4����笠�����ˮ�������ԣ�����������Ũ�ȴ�С˳��Ϊ��c(NH4+)>c(SO42-)>c(H+)>c(OH-)��
�ʴ�Ϊ��c(NH4+)>c(SO42-)>c(H+)>c(OH-)��

 Сѧ������ҵϵ�д�
Сѧ������ҵϵ�д� ��ʿһ��ȫͨϵ�д�
��ʿһ��ȫͨϵ�д�����Ŀ������ʵ�����Ӧ�Ľ��ͻ������ȷ����( )
ѡ�� | ʵ�� | ���ͻ���� |
A |
2 mL 0.2mol L-1 H2C2O4(����)��Һ | �Ҳ��Թ�����Һ��ɫ��ȥ������֪��Ӧ��Ũ��Խ��Ӧ����ԽС |
B | �ֱ���ʢ�е���ú�͡���ˮ�Ҵ������ձ��м����С��ȵĽ����ƣ��Աȹ۲����� | �Ҵ��������ǻ��ϵ���ԭ�ӻ��� |
C | �ֱ���ʢ�ж����͡�ʯ�������ձ��м��������ռ���Һ����ּ��ȣ���ȴ | �����͡�ʯ�����ܷ���������Ӧ |
D | ����ʯ��ʳ��ˮ��Ӧ���ɵ����壬ͨ�����Ը��������Һ�У��۲���Һ��ɫ�仯 | ����Һ��ɫ��ȥ��֪��Ȳ�ܷ���������Ӧ |
A. AB. BC. CD. D
����Ŀ��ijС��Ϊ̽��K2Cr2O7��Cr�ڲ�ͬ�����´��ڵ���Ҫ��ʽ�������ص㡣������(��ϵ��ʵ��I��ii��)����������ϵ��ʵ�飺
ϵ��ʵ��I | װ�� | �ι��е��Լ� | �Թ��е��Լ� | ���� | ���� |
i |
| 1 mLˮ | 4mL 0.1 mol��L-1 K 2Cr2O7 ��ɫ��Һ | �� | ��Һ��ɫ����dz |
ii | 1 mLˮ | �� 60��ˮԡ | ��Һ��ɫ��i���Ա�dz | ||
iii | 1 mL 18.4 mol��L-1 Ũ���� | �� | ��Һ��ɫ��i���Ա��� | ||
iv | 1 mL 6 mol��L-1 NaOH ��Һ | �� | _____________________ | ||
v | 3��ŨKI��Һ | iv����Һ | �� | ���������� | |
vi | ����ϡ���� | v����Һ | �ߵα��� | ��Һ��ɫ�ɻ�ɫ���ɫ������ī��ɫ |
��֪��K2CrO4��ҺΪ��ɫ��Cr3+��ˮ��Һ��Ϊ��ɫ��
�밴Ҫ��ش��������⣺
(1)д��K2C2O7������������ƽ��ת�������ӷ���ʽ��________________________���Ա�ʵ��i��ii���ɵý����Ǹ�ת����Ӧ�ġ�H______0(��>������<��)��
(2)���ʵ��i��ii����������ii��������ֵ���Ҫ������__________________��
(3)�Ʋ�ʵ��iv��ʵ������Ϊ________________________���Ա�ʵ��i��ii��iv��ʵ������֪��������K2Cr2O7��Cr�ڼ�����������Ҫ��______������ʽ���ڡ�
(4)�Ա�ʵ��v��vi����֪����______�����£�+6��Cr����ԭΪ______��
(5)Ӧ������ʵ����ۣ���һ��̽����Cr2O72-��ˮ��Ʒ�õ�ⷨ����Ч����Ӱ�����أ�ʵ�������±���ʾ(Cr2O72-����ʼŨ�ȡ��������ѹ�����ʱ��Ⱦ���ͬ)��
ϵ��ʵ��� | i | ii | iii | iv |
��Ʒ���Ƿ��Fe2(SO4)3 | �� | �� | ����5 g | �� |
��Ʒ���Ƿ����ϡ���� | �� | ����1 mL | ����1 mL | ����1 mL |
�缫���� | ����������Ϊʯī | ����Ϊʯī������Ϊ�� | ||
Cr2O72- | 0.922 | 12.7 | 20.8 | 57.3 |
��ʵ����Cr2O72-�������ķ�Ӧʽ��_________________________________________________��

��ʵ��i��Fe3+ȥ��Cr