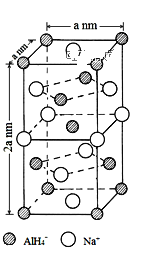
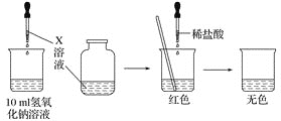
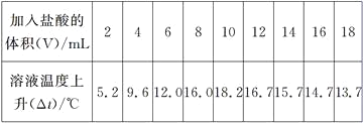
��Ŀ����
����Ŀ��pC����pH����ָ��Һ���������ʵ���Ũ�ȵij��ö����ĸ�ֵ����ij��Һ��Ũ��Ϊ1��10��2mol/L������Һ�и����ʵ�pC=-lg1��10��2=2����֪H2RO3��Һ�д��ڵĻ�ѧƽ��Ϊ��
RO2(g)+H2O ![]() H2RO3
H2RO3 ![]() H+ +HRO3- ��HRO3-
H+ +HRO3- ��HRO3-![]() H++RO32-����ͼΪH2RO3������Һ��pC-pHͼ����ش��������⣺
H++RO32-����ͼΪH2RO3������Һ��pC-pHͼ����ش��������⣺
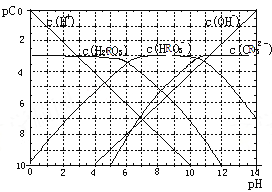
��1����pH=2��4ʱ��H2RO3��Һ����Ҫ���ڵ�����Ϊ��_____��
��2��H2RO3һ������ƽ�ⳣ������ֵKa1��_______��
��3����֪��298Kʱ��H2RO3�ĵ��볣��Ka2=5.6��10��11���۲���ͼ�ж�NaHRO3��Һ��_______�ԣ���ͨ�����㣬���õ��롢ˮ��ƽ�ⳣ��˵������____________��
��4��һ��Ũ�ȵ�NaHRO3��Na2RO3�����Һ��һ�֡�������Һ������������Һ�м���������ǿ���ǿ���Һ��pH�仯������ԭ����_________ ��
��5��һ���¶��£�������MRO3(M��Mg2+��Ca2+��Mn2+)�ij����ܽ�������ͼ��ʾ����֪��p(M2+)=-lg c(M2+)��p(RO32-)= -lgc(RO32-)
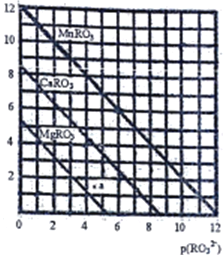
��MgRO3��CaRO3��MnRO3��Ksp�ɴ�С��˳��Ϊ__________________��
�� a ��ʱc(Mg2+)_____ c(RO32-) ���>����<����=��������ʱMgRO3��Һ__________������ﱥ�͡���δ�ﱥ�͡���
��������ij��Һ�е�Mn2+��MnRO3�ε���ʽ������ȫ����Һ��Mn2+���ӵ�Ũ��С��l��10-5mol/L�����������Һ�е�p(RO32-)�ķ�Χ��_________________��
���𰸡� H+��HRO3- 1��10-6 ���� Kh=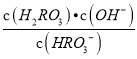 =
=![]() =
=![]() =10-8��Ka2��HRO3-ˮ��̶ȴ��ڵ���̶ȣ�����Һ�ʼ��ԡ� �ڸ���Һ�м����������ᣬʹƽ�⣺HRO3-
=10-8��Ka2��HRO3-ˮ��̶ȴ��ڵ���̶ȣ�����Һ�ʼ��ԡ� �ڸ���Һ�м����������ᣬʹƽ�⣺HRO3-![]() H++RO32-�����ƶ��������Һ��H+Ũ�����Ӻ��٣�������Һ�м��������ļʹƽ��HRO3-
H++RO32-�����ƶ��������Һ��H+Ũ�����Ӻ��٣�������Һ�м��������ļʹƽ��HRO3-![]() H++RO32-�����ƶ��������Һ��OH-Ũ��Ҳ���Ӳ��� Ksp��MgRO3����Ksp��Ca RO3����Ksp��Mn RO3�� �� δ�ﱥ�� ��7
H++RO32-�����ƶ��������Һ��OH-Ũ��Ҳ���Ӳ��� Ksp��MgRO3����Ksp��Ca RO3����Ksp��Mn RO3�� �� δ�ﱥ�� ��7
����������1������ͼ���֪��pH=2��4ʱ��H2RO3��Һ����Ҫ���ڵ�����ΪH+��HRO3-����2������ͼ���֪Ph=6ʱ��H2RO3��HRO3-��Ũ����ȣ���H2RO3һ������ƽ�ⳣ������ֵKa1��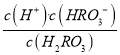 ��10��6����3������Kh=
��10��6����3������Kh=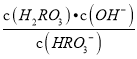 =
=![]() =
=![]() =10-8��Ka2����˵��HRO3-ˮ��̶ȴ��ڵ���̶ȣ�����Һ�ʼ��ԡ���4�������ڸ���Һ�м����������ᣬʹƽ�⣺HRO3-
=10-8��Ka2����˵��HRO3-ˮ��̶ȴ��ڵ���̶ȣ�����Һ�ʼ��ԡ���4�������ڸ���Һ�м����������ᣬʹƽ�⣺HRO3-![]() H++RO32-�����ƶ��������Һ��H+Ũ�����Ӻ��٣�������Һ�м��������ļʹƽ��HRO3-
H++RO32-�����ƶ��������Һ��H+Ũ�����Ӻ��٣�������Һ�м��������ļʹƽ��HRO3-![]() H++RO32-�����ƶ��������Һ��OH-Ũ��Ҳ���Ӳ������Կ���������Һ����5���ٸ���ͼ���֪����Һ��p(RO32-)���ʱ��p(Mn2+)��С������MnRO3���ܶȻ�������С����MgRO3��CaRO3��MnRO3��Ksp�ɴ�С��˳��ΪKsp��MgRO3����Ksp��CaRO3����Ksp��MnRO3������ a����MgRO3�����Ϸ�����ʱp(Mg2+)��p(RO32-)��������Һ��c(Mg2+)��c(RO32-) ����ʱŨ����С���ܶȻ�������MgRO3��Һδ�ﱥ�ͣ��۸���ͼ���֪Ksp��MnRO3����1��10��12��������ij��Һ�е�Mn2+��MnRO3�ε���ʽ������ȫ����Һ��Mn2+���ӵ�Ũ��С��l��10-5mol/L�����������Һ��c(RO32-)��l��10-7mol/L��������Һ��p(RO32-)�ķ�Χ��С��7��
H++RO32-�����ƶ��������Һ��OH-Ũ��Ҳ���Ӳ������Կ���������Һ����5���ٸ���ͼ���֪����Һ��p(RO32-)���ʱ��p(Mn2+)��С������MnRO3���ܶȻ�������С����MgRO3��CaRO3��MnRO3��Ksp�ɴ�С��˳��ΪKsp��MgRO3����Ksp��CaRO3����Ksp��MnRO3������ a����MgRO3�����Ϸ�����ʱp(Mg2+)��p(RO32-)��������Һ��c(Mg2+)��c(RO32-) ����ʱŨ����С���ܶȻ�������MgRO3��Һδ�ﱥ�ͣ��۸���ͼ���֪Ksp��MnRO3����1��10��12��������ij��Һ�е�Mn2+��MnRO3�ε���ʽ������ȫ����Һ��Mn2+���ӵ�Ũ��С��l��10-5mol/L�����������Һ��c(RO32-)��l��10-7mol/L��������Һ��p(RO32-)�ķ�Χ��С��7��

 ��1����Ԫ�¿�������ĩϵ�д�
��1����Ԫ�¿�������ĩϵ�д�����Ŀ���������ֽ�ˮ�����������һ����Ҫ���л��ϳ�ԭ�ϡ�ij��ѧС����ˮ����(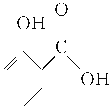 )�ͼ״������Դ������ºϳ�ˮ�����������������ʡ�
)�ͼ״������Դ������ºϳ�ˮ�����������������ʡ�
ʵ�鲽�裺
��.��ͼ����������ƿ�м���13.8 g(0.1 mol)ˮ�����24 g(30 mL,0.75 mol)�״����������м���Լ10 mL�ױ�(�ױ���ˮ�γɹ�����е�Ϊ85 ������ʵ���м���ױ�����ˮ����)����С�ĵؼ���5 mLŨ���ᣬҡ�����ȣ�����1��2����ʯ����װ��ʵ��װ�ã���85��95 ���º��¼��ȷ�Ӧ1.5Сʱ��
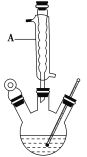
��.��װ����ȴ������״���Ȼ��ת������Һ©��������������ˮ��5% NaHCO3��Һ��ˮϴ�ӣ�������IJ������������ˮMgSO4���壬���˵õ�������
��.���������������ռ�221��224 ������֣���ˮ�������9.12 g��
��������������
���� | ������ | ��ɫ״̬ | ����ܶ� (g��cm��3) | �۵� (��) | �е� (��) |
ˮ���� ���� | 152 | ��ɫҺ�� | 1.18 | ��8.6 | 218�� 224 |
ˮ���� | 138 | ��ɫ���� | 1.44 | 158 | 210 |
�״� | 32 | ��ɫҺ�� | 0.792 | ��97 | 64.7 |
�����������Ϣ�ش��������⣺
��1������A��������________�������ʯ��������_____________________�������Ⱥ���δ�ӷ�ʯ��Ӧ��ȡ����ȷ������________________________________________________��
��2���Ʊ�ˮ�������ʱ������ʵļ��ȷ�����____________________________________________��
��3��ʵ���м���ױ��Ժϳ�ˮ���������������___________________________________________��
��4����Ӧ����������״����õķ�����__________________________________________��
��5��ʵ���м�����ˮ����þ��������__________________________________________����ʵ��IJ���Ϊ________(������λ��Ч����)��