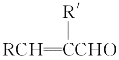��Ŀ����
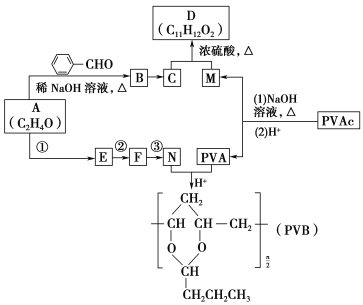
����Ŀ���⻯���ƣ�NaAlH4����һ���������ʴ�����ϣ���������Ti��NaAlH4��150��ʱ���⣬��170�桢15. 2MPa���������ظ����⡣NaAlH4����AlCl3��NaH���ʵ������ºϳɡ�NaAlH4�ľ����ṹ��ͼ��ʾ��

��1����̬Tiԭ�ӵļ۵��ӹ���Ų�ͼΪ___________��
��2��NaH���۵�Ϊ800�棬�������л��ܼ���NaH����_____���壬�����ʽΪ____________��
��3��AlCl3��178��ʱ����������������Է�������ԼΪ267���������ӵĽṹʽΪ______________ ��������λ����
��4��AlH4���У�Al�Ĺ���ӻ���ʽΪ_______��������AlH4���ռ乹����ͬ����������_________���ѧʽ����
��5��NaAlH4�����У���Na+�����ҵȾ��AlH4����______����NaAlH4������ܶ�Ϊ______________g��cm��3���ú�a�Ĵ���ʽ��ʾ����
���𰸡�![]() ����
���� ![]()
![]() sp3 NH4+��BH4����SO42�� ��PO43�� �����������𰸣� 8
sp3 NH4+��BH4����SO42�� ��PO43�� �����������𰸣� 8 ![]() ����
���� ![]() ����
����
��������
��1��Tiԭ�Ӻ��������Ϊ22���۵����Ų�ʽΪ3d24s2���۵����Ų�ͼΪ![]() ��
��
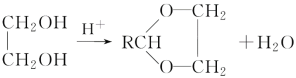
��2��NaH���۵�Ϊ800�棬�������л��ܼ���Ӧ�������Ӿ��壬�����������⸺���ӹ��ɣ�����ʽΪ![]() ��
��
��3���Ȼ�����178��ʱ�������۷е�ϵͣ����ڷ��Ӿ��壬��������Է�������ԼΪ267���������ӵķ���ʽΪAl2Cl6����ԭ����������ֻ��3�����ӣ��γ�3�����ۼ���ÿ����ԭ�Ӻ��ĸ���ԭ���γɹ��ۼ���������һ�����õ��Ӷ�����ԭ���ṩ�γɵ���λ�����ṹʽ��ͼ![]() ��
��
��4��AlH4-��Al�Ĺ���ӻ���ĿΪ4+![]() =4��Al��ȡsp3�ӻ���Ϊ�������幹�ͣ���AlH4-�ռ乹����ͬ����������ΪNH4+��SO42-�ȣ�
=4��Al��ȡsp3�ӻ���Ϊ�������幹�ͣ���AlH4-�ռ乹����ͬ����������ΪNH4+��SO42-�ȣ�
��5�����ݾ�̯����֪��������AlH4-��ĿΪ1+8��![]() +4��
+4��![]() =4��Na+��ĿΪ6��
=4��Na+��ĿΪ6��![]() +4��
+4��![]() =4���������λ��Ϊ1��1�������ĵ�AlH4-�о�����֮�����ҵȾ��Na+λ�ھ�����֮�䡢�������������������Ҳ������ġ�����������������ǰ��������ģ���AlH4-�����ҵȾ��Na+��8��������Na+�����ҵȾ��AlH4-��8������������Ϊ4��
=4���������λ��Ϊ1��1�������ĵ�AlH4-�о�����֮�����ҵȾ��Na+λ�ھ�����֮�䡢�������������������Ҳ������ġ�����������������ǰ��������ģ���AlH4-�����ҵȾ��Na+��8��������Na+�����ҵȾ��AlH4-��8������������Ϊ4��![]() g�������ܶ�Ϊ
g�������ܶ�Ϊ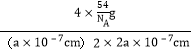 =
=![]() gcm-3��
gcm-3��

 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
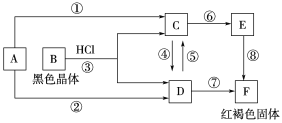
Сѧ��10����Ӧ����ϵ�д�����Ŀ����ͼ��ʾװ�ü�����ϩʱ����Ҫ���ӵ���

��ϩ���Ʊ� | �Լ�X | �Լ�Y | |
A | CH3CH2Br��NaOH�Ҵ���Һ���� | H2O | KMnO4������Һ |
B | CH3CH2Br��NaOH�Ҵ���Һ���� | H2O | Br2��CCl4��Һ |
C | CH3CH2OH��ŨH2SO4������170�� | NaOH��Һ | KMnO4������Һ |
D | CH3CH2OH��ŨH2SO4������170�� | NaOH��Һ | Br2��CCl4��Һ |