��Ŀ����
����Ŀ��A�IJ����Ǻ���һ������ʯ�ͻ�����չˮƽ�ı�־��B��D�����������ֳ������л��F�Ǹ߷��ӻ�����ת����ϵ��ͼ��ʾ��

��֪��RCHO ![]() RCOOH
RCOOH
��1��A���ӵĽṹ��ʽΪ______��D�й���������Ϊ______��
��2���ڷ�Ӧ�١����У����ڼӳɷ�Ӧ����______������ȡ����Ӧ����______(�����)��
��3��B��D��Ӧ���бȽϻ�������߸÷�Ӧ���ʵķ�����Ҫ��______��______�������ᴿ������е�G��ѡ�õĵ��Լ�Ϊ______��ʵ�����Ϊ__________��
��4��д�����з�Ӧ�Ļ�ѧ����ʽ��
��B��C��____________��
��B��D��G��______________��
��A��F��________________��
���𰸡�CH2=CH2 �Ȼ� �� �� ���� ʹ��Ũ���������� ����̼������Һ ��Һ 2CH3CH2OH+O2 ![]() 2CH3CHO + 2H2O CH3COOH+C2H5OH
2CH3CHO + 2H2O CH3COOH+C2H5OH![]() CH3COOC2H5+H2O nCH2=CH2
CH3COOC2H5+H2O nCH2=CH2 ![]()
��������
���ݳ����л�����ת��������𣻸��ݳ����л���Ļ�ѧ���ʷ������
A�IJ����Ǻ���һ������ʯ�ͻ�����չˮƽ�ı�־��AΪ��ϩ������֪������ת����ϵͼ��֪����ϩ��ˮ�����ӳɷ�Ӧ�����Ҵ�����BΪ�Ҵ����Ҵ��������ڴ����������·�Ӧ������ȩ����CΪ��ȩ����ȩ������������Ӧ�������ᣬ��DΪ���ᣬ�������Ҵ���Ũ������ȵ������·���������Ӧ����������������GΪ������������ϩ��һ�������·����Ӿ۷�Ӧ���ɾ���ϩ����FΪ����ϩ��
��1��AΪ��ϩ����A���ӵĽṹ��ʽΪCH2=CH2��DΪ���ᣬ����Ĺ�����Ϊ�Ȼ���
��2���ڷ�Ӧ�������У����ڼӳɷ�Ӧ���� ��������ȡ����Ӧ���� ����
��3��B��D��Ӧ���бȽϻ�������߸÷�Ӧ���ʵķ�����Ҫ�У�ʹ��Ũ���������������ȵȣ���������Ҵ�����������Ӧ�õ������������л������ᡢ�Ҵ����ñ���̼������Һ�ɽ��������������ܽ�ȣ�ʹ���������ֲ�������ͬʱ�����Ҵ�����ȥ���ᣬ������ᴿ������е�����������ѡ�õ��Լ�Ϊ����̼������Һ�����ֲַ������ϲ���״Һ��Ϊ�������������з�Һ�������ɷ��룻
��4�����Ҵ��������ڴ����������·�Ӧ������ȩ������ʽΪ��2CH3CH2OH+O2 ![]() 2CH3CHO + 2H2O��
2CH3CHO + 2H2O��
���Ҵ���������Ũ������ȵ������·�Ӧ��������������ˮ������ʽΪ��CH3COOH+C2H5OH![]() CH3COOC2H5+H2O��
CH3COOC2H5+H2O��
����ϩ��һ�������·����Ӿ۷�Ӧ���ɾ���ϩ������ʽΪ��nCH2=CH2 ![]() ��
��

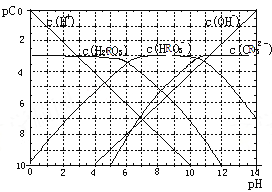
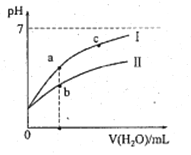
����Ŀ���±�Ϊ25��ʱijЩ����ĵ���ƽ�ⳣ�����±�ͼ���ʾ25��ʱ��ϡ��CH3COOH��HClO�������ϡ��Һ����ҺpH���ˮ���ı仯�������£��й�˵����ȷ����
����ĵ���ƽ�ⳣ��(25��) | |
CH3COOH | HClO |
Ka=1.8��10-5 | Ka=3.0��10-8 |
A. ͼ���У�a�������Ũ��>b�������Ũ��
B. ͼ���У�c(H+)��c(R-)��ֵ��a��>c��(HR����CH3COOH��HClO)
C. pH��ͬ��CH3COONa��Һ��NaClO��Һ��Ũ�ȹ�ϵ:c(CH3COOHNa)<c(NaClO)
D. ����ҺŨ����ȣ�CH3COONa��Һ��c(OH-)+c(CH3COO-)>NaClO��Һ��c(OH-)+c(C1O-)