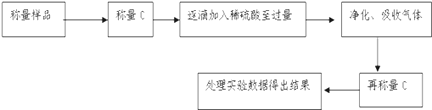
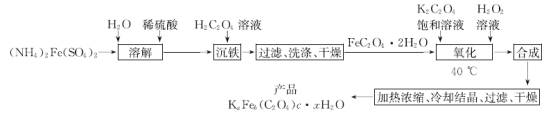
��Ŀ����
����Ŀ��2019��3��21���ǵڶ�ʮ�߽�������ˮ����������ˮ��Դ���������÷�ˮ��ʡˮ��Դ����ǿ��ˮ�Ļ��������ѱ�Խ��Խ���������ע����֪��ij��ɫ��ˮ�п��ܺ���H+��NH4+��Fe3+��Al3+��Mg2+��Na+��NO3-��CO32-��SO42-�еļ��֣�Ϊ������ɷ֣��ֱ�ȡ��ˮ��Ʒ100![]() ������������ʵ�飬��������й�ͼ������ͼ��ʾ����ش��������⣺
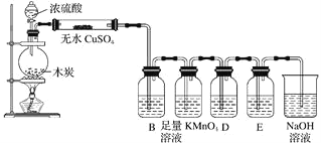
������������ʵ�飬��������й�ͼ������ͼ��ʾ����ش��������⣺

(1)��������3��ʵ����Է�����ˮ��һ�������ڵ���������_______��һ�����ڵ���������____________________��
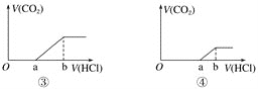
(2)д��ʵ���ͼ���г����ﵽ��������������ٷ����仯�η�����Ӧ�����ӷ�Ӧ����ʽ��_________________________��
(3)����ͼ����ԭ��Һ��c(NH4+)��c(Al3+)�ı�ֵΪ__________�����ó��������������__________g��
(4)��ͨ��ʵ��ȷ��ԭ��ˮ��c(Na+)=0.14mol/L�����ж�ԭ��ˮ��NO3-�Ƿ����?__________(��������������������������ȷ����)�������ڣ�c(NO3-)=__________mol/L��(�������ڻ�ȷ����˿ղ���)��
���𰸡�CO32- Na+��H+��Al3+��NH4+ NH4++OH-=NH3��H2O 1��1 0.546 ���� 0.36
��������
(1)����ʵ�����ɫ��ӦΪ��ɫȷ������Na+������ʵ��ڼ���ϡHCl�ữ��BaCl2��Һ��������ɫ������ȷ������SO42-������ʵ��ۼ���NaOH��Һ����ʼ������˵������H+������H+��CO32-���ܴ������棬֤��������CO32-������������ɫ������֤�������в������ɫ������Fe3+��Ȼ��������ٱ仯����������ȫ��ʧ��֤������Al3+����Mg2+���ɼ�ͨ���ۿ�֤������H+��Al3+��NH4+����CO32-��Mg2+��Fe3+��Ȼ����ݷ�Ӧ���������ĵ�NaOH�����ʵ���ȷ���������ӵ����ʵ����Ķ��٣������Һ������ȷ���������ӵĴ��ڼ���Ũ�ȴ�С��
(1)����ʵ���ȷ������Na+������ʵ���ȷ������SO42-������ʵ���ȷ����H+��Al3+��NH4+��û��Fe3+��Mg2+����ΪCO32-��H+��Al3+���ܹ��棬����һ��������CO32-���ɼ��÷�ˮ��һ�������ڵ���������CO32-��һ�����ڵ���������Na+��H+��Al3+��NH4+��
(2)ʵ���ͼ���г����ﵽ��������������ٷ����仯�Σ���NH4+��OH-֮������ӷ�Ӧ�����ӷ���ʽΪ��NH4++OH-=NH3��H2O��
(3)����ͼ�ӿ�ʼ�γɳ����������ﵽ���ֵ��������ӦAl3++3OH-=Al(OH)3����n(Al3+)=![]() =0.007mol��n(NH4+)=0.042mol-0.035mol=0.007mol����Һ�������ͬ������ԭ��Һ��c(NH4+)��c(Al3+)�ı�ֵΪ1��1�����ó������������m[Al(OH)3]=0.007mol��78g/mol=0.546g��
=0.007mol��n(NH4+)=0.042mol-0.035mol=0.007mol����Һ�������ͬ������ԭ��Һ��c(NH4+)��c(Al3+)�ı�ֵΪ1��1�����ó������������m[Al(OH)3]=0.007mol��78g/mol=0.546g��
(4)�������ᱵ����������2.33g��n(SO42-)=n(BaSO4)=![]() =0.01mol�����ݵ���غ㣬�����ӵ�������ʵ���Ϊ0.01mol��2=0.02mol�������ӵ�������ʵ���Ϊn(H+)+n(Al3+)+ n(NH4+)+n(Na+)=0.014mol+0.007��3mol+0.007mol+0.14mol/L��0.1L=0.056mol��������������ڸ��������������ԭ��ˮ�д���NO3-��c(NO3-)=
=0.01mol�����ݵ���غ㣬�����ӵ�������ʵ���Ϊ0.01mol��2=0.02mol�������ӵ�������ʵ���Ϊn(H+)+n(Al3+)+ n(NH4+)+n(Na+)=0.014mol+0.007��3mol+0.007mol+0.14mol/L��0.1L=0.056mol��������������ڸ��������������ԭ��ˮ�д���NO3-��c(NO3-)=![]() =0.36mol/L��
=0.36mol/L��

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�