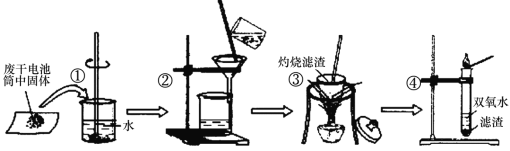
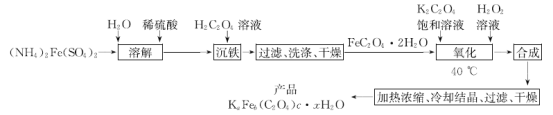
��Ŀ����
����Ŀ��ʵ�����Ʊ���������KaFeb(C2O4)c��xH2O�Ĺ����������£�
�ش��������⣺
(1)���ܽ���ʱ��Ϊʹ���õ�ˮ�в���O2�����õIJ���������_________________��
(2)��H2C2O4(����)��������ʱ����Ӧ�����ӷ���ʽΪ______________��
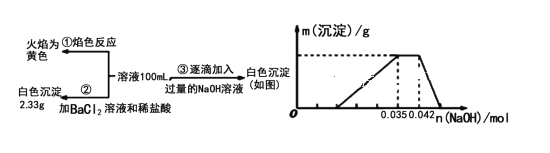
(3)FeC2O4��2H2O�ȷֽ������ռ��¶��йأ���N2�������ȷֽ�ʱ������IJ�����(������Ʒ��ʣ������/������Ʒ����ʼ������100��)���¶ȵĹ�ϵ��ͼ��ʾ����B��C�ı仯�У�������Ӧ�Ļ�ѧ����ʽΪ___________________��
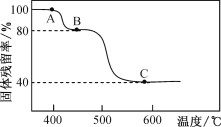
(4)��������ʱ���¶Ȳ��˳���40�棬��ԭ����_______��
(5)Ϊ�ⶨ��ƷKaFeb(C2O4)c��xH2O(��Ԫ��Ϊ��3��)����ɣ���ȡ��Ʒ0.2455g�������ܽ����0.02000 mol��L��1��KMnO4����Һ�ζ����յ㣬���ı���Һ30.00 mL���������ζ���C2O42���ı���Һ�м�������п�ۣ���������ɫ��ʧ������ϴ�ӣ���Һ��ϴ��Һ����0.02000 mol��L��1��KMnO4����Һ�ζ����յ㣬���ı���Һ5.00 mL����ò�Ʒ�Ļ�ѧʽΪ____________��
���𰸡���� H2C2O4+Fe2++2H2O= FeC2O42H2O��+2H+ FeC2O4![]() FeO+CO2��+CO�� ����˫��ˮ�ķֽ���ʧ K3Fe(C2O4)3��3H2O
FeO+CO2��+CO�� ����˫��ˮ�ķֽ���ʧ K3Fe(C2O4)3��3H2O
��������
(1)���ܽ���ʱ��Ϊʹ���õ�ˮ�в���O2��������������ܽ�����¶����߶���С���д�����(2)��H2C2O4(����)��������ʱ��H2C2O4����Һ�е�Fe2+������Ӧ������FeC2O42H2O������
(3) ����FeC2O4��2H2OΪ1mol����180g���ᾧˮ�������Ⱥ�ͨ����ʧȥ�ᾧˮ��Ȼ����ˮ���ٷֽ⡣��ͼ��B��C������гɷ���֤��B��ӦΪ��������ˮ�Σ���1mol FeC2O4������Ϊ144g��![]() ��C��ӦΪFeO������Ϊ72g��
��C��ӦΪFeO������Ϊ72g��![]() ���Ӷ�ȷ��B��ɷ�ΪFeC2O4��C��ɷ�ΪFeO��
���Ӷ�ȷ��B��ɷ�ΪFeC2O4��C��ɷ�ΪFeO��
(4)��������ʱ����Ϊ˫��ˮ�ֽ⣬����������¶Ȳ��˳���40����
(5)����ʹ��KMnO4����Һ����һ��������KMnO4����Һ����C2O42�����ڶ���������Zn��Fe3+��ԭΪFe2+��������KMnO4����Һ����Fe2+���Ӷ������C2O42����Fe2+�����ʵ��������õ���غ㣬���K+�����ʵ��������������غ㣬����ᾧˮ�����ʵ�����
(1)���ܽ���ʱ��Ϊʹ���õ�ˮ�в���O2�����õIJ�����������С���Ϊ����У�
(2)��H2C2O4(����)��������ʱ��H2C2O4��Fe2+��Ӧ������FeC2O42H2O��������Ӧ�����ӷ���ʽΪH2C2O4+Fe2+= FeC2O4��+2H+����Ϊ��H2C2O4+Fe2++2H2O= FeC2O42H2O��+2H+��
(3) ����FeC2O4��2H2OΪ1mol����180g����ͼ��B��C������гɷ���֤��B��ӦΪ��������ˮ�Σ���1mol FeC2O4������Ϊ144g��![]() ��C��ӦΪFeO������Ϊ72g��
��C��ӦΪFeO������Ϊ72g��![]() ���Ӷ�ȷ��B��ɷ�ΪFeC2O4��C��ɷ�ΪFeO����B��C�ı仯�У�������Ӧ�Ļ�ѧ����ʽΪFeC2O4
���Ӷ�ȷ��B��ɷ�ΪFeC2O4��C��ɷ�ΪFeO����B��C�ı仯�У�������Ӧ�Ļ�ѧ����ʽΪFeC2O4![]() FeO+CO2��+CO������Ϊ��FeC2O4
FeO+CO2��+CO��������FeC2O4![]() FeO+CO2��+CO����
FeO+CO2��+CO����
(4) ��������ʱ���¶Ȳ��˳���40�棬��ԭ���Ǽ���˫��ˮ�ķֽ���ʧ����Ϊ������˫��ˮ�ķֽ���ʧ��
(5)��5C2O42��+2MnO4-+16H+=10CO2��+2Mn2++8H2O
n(C2O42��)=![]() ��0.02000 mol��L��1��30.00mL��10-3L/mL1.500��10-3mol
��0.02000 mol��L��1��30.00mL��10-3L/mL1.500��10-3mol
��5Fe2++MnO4-+8H+=5Fe3++Mn2++4H2O
n(Fe2+)= n(Fe3+)=5��0.02000 mol��L��1��5.00mL��10-3L/mL=5.000��10-4mol
���ݵ���غ㣺n(K+)+3n(Fe3+)=2n(C2O42��)
n(K+)=1.500��10-3mol
n(H2O)=
![]() ��a:b:c:x= n(K+):n(Fe3+):n(C2O42��):n(H2O)=3:1:3:3
��a:b:c:x= n(K+):n(Fe3+):n(C2O42��):n(H2O)=3:1:3:3
��ѧʽΪK3Fe(C2O4)3��3H2O����ΪK3Fe(C2O4)3��3H2O��

 �γ̴����Ծ�����100��ϵ�д�
�γ̴����Ծ�����100��ϵ�д� �¾�����ĩ���100��ϵ�д�
�¾�����ĩ���100��ϵ�д� ȫ�ܴ���100��ϵ�д�
ȫ�ܴ���100��ϵ�д�����Ŀ��ijʵ��С���Ʊ����ᾧ��(H2C2O4��2H2O)��ʵ��װ�����£�
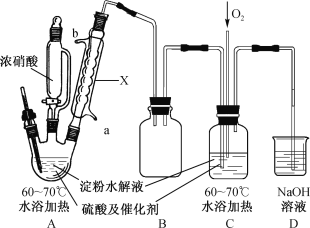
��֪��I.����(H2C2O4)�Ƕ�Ԫ���ᣬ�۵�101��102�棻
II.������ܽ�����±���ʾ��
�¶�/�� | 20 | 30 | 40 | 50 | 60 | 70 |
�ܽ��/g��(100gˮ)��1 | 9.5 | 14.3 | 21.2 | 31.4 | 46.0 | 84.5 |
III.�ڴ�������������£���Ũ������������ˮ��Һ���Ʊ����ᣬ��������Ҫ��ӦΪ��
C6H12O6��12HNO3��3H2C2O4��9NO2����3NO����9H2O��
�ش��������⣺
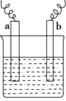
(1)װ��A������X����Ϊ___________��ˮ�ӽӿ�___________(����a������b��)���롣
(2)װ��B��������___________�����װ��C��Ŀ����_________________________��
(3)��ӦC6H12O6��12HNO3��3H2C2O4��9NO2����3NO����9H2O�У�ÿ����1 mol H2C2O4ת�Ƶ��ӵ����ʵ���Ϊ____________��
(4)��װ��A��C�з�ӦҺŨ�������ýᾧ�����˵ôֲ��ᾧ�壬�ᴿH2C2O4��2H2O�ķ�����_________________________��
(5)̽����������ʣ�
����Na2CO3��Һ�еμ�H2C2O4��Һ�������������壬˵����������Ա�̼��________(����ǿ����������)��
����NaClO��Һ�м������H2C2O4��Һ�������ݲ������ܽ�����һ��������ӷ���ʽΪ___��