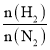��Ŀ����
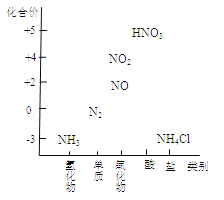
����Ŀ��������������������������Ź㷺��Ӧ�ã�ij��ѧ��ȤС������ͼһװ��̽���������й����ʡ�
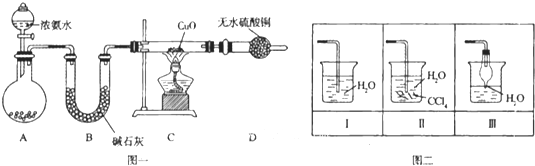
��1��װ��A����ƿ���Լ���ѡ�� �����������B��������
a����ʯ�� b��Ũ���� c����ʯ�� d���ռ���Һ
��2�����Ӻ�װ�ò�����װ�õ������Ժ�װ��ҩƷ��Ȼ��Ӧ�� ����I�������
�����������Բ����ƿ�м��백ˮ ����װ��C
��3��ʵ���й۲쵽C��CuO��ĩ��죬D����ˮ����ͭ���������ռ���һ�ֵ������壬��÷�Ӧ��ػ�ѧ����ʽΪ ,���÷�Ӧ֤���������� �ԣ�
��4����ʵ��ȱ��β������װ�ã�ͼ��������������β����װ���� ����װ���������
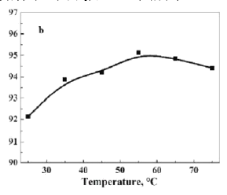
��5��������������ˮ������״���£���2.24L�İ�������ˮ���0.5L��Һ��������Һ�����ʵ���Ũ��Ϊ mol/L��
���𰸡���1��ac������ˮ�������ﰱ�� ����2������3��3CuO+2NH3![]() 3Cu+N2+3H2O����ԭ����4����������5��0.2��
3Cu+N2+3H2O����ԭ����4����������5��0.2��
��������
�����������1��Aװ���Ʊ�����������Ũ��ˮ�����ӷ����ص㣬��ƿ��ʢ�ż�ʯ�Һ���ʯ�һ��������ƹ��壬���ac��ȷ��Bװ�õ������Ǹ��ﰱ������2����ͨһ��ʱ��İ������ų�װ���еĿ�����Ȼ���ȼ�ƾ��ƣ���I��ȷ����3��CuO���˵��ת����ͭ����ˮ����ͭ������˵������H2O����˷�Ӧ����ʽΪ3CuO+2NH3![]() 3Cu+N2+3H2O��������N�Ļ��ϼ����ߣ�������������ԭ������4��������������ˮ����ֹ���������ѡ�õ���II��III����5����Һ���ʵ���Ũ��Ϊ2.24/22.4/0.5mol��L��1=0.2mol��L��1��
3Cu+N2+3H2O��������N�Ļ��ϼ����ߣ�������������ԭ������4��������������ˮ����ֹ���������ѡ�õ���II��III����5����Һ���ʵ���Ũ��Ϊ2.24/22.4/0.5mol��L��1=0.2mol��L��1��

 ��У����ϵ�д�
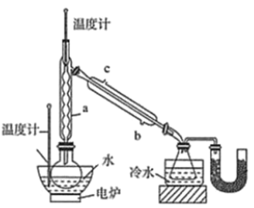
��У����ϵ�д�����Ŀ�����⻯��(NaBH4)ͨ��Ϊ��ɫ��ĩ��������ˮ���⣬�����������(�۵㣺-101�棬�е㣺33��)�����л��ϳ��б���Ϊ�����ܻ�ԭ�������Ʊ����⻯�Ƶ�������ͼ��
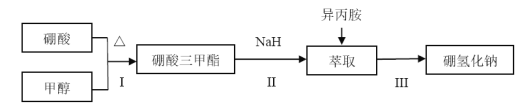
�������������Ʊ���������(H3BO3)�������״�����Բ����ƿ�У�Ȼ�����ؼ���Ũ���ᣬ������ƿ�ϼ�װ������a���õ�¯��ˮԡ�����ȣ�����2Сʱ���ռ�������������״�����Һ��װ����ͼ��ʾ(�г�װ����ȥ����ͬ)��
����������B(OCH3)3 | �״� | �״��� | |
�ܽ��� | ���Ҵ����״����ܣ���ˮ�� | ��ˮ���� | ���ڼ״�������������� |
�е�/�� | 68 | 64 | 450 |
������������״������Ĺ��е�Ϊ54�� | |||
(1)ֱ����������ȴˮӦ��________(����b������c��)�ӿڽ��롣
(2)Ũ�����������_______��
(3)��ʵ�����ˮԡ���ȣ��ŵ���________��U�����Լ���������__________��
(4)��240�������½��У��������������⻯�Ʒ�Ӧ��ȡNaBH4��ͬʱ����CH3ONa��д���÷�Ӧ�Ļ�ѧ����ʽ______��
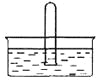
(5)��ȡʱ�ɲ���������ȡ������װ����ͼ��ʾ��ʵ��ʱ��ƿ���ܼ����������������ص���2���������������ܣ������������ֽ��Ͳ1�У�������ȡ����ȡҺҺ��ﵽ������3����ʱ����������3������ƿ���Ӷ�ʵ��������ȡ������ȡ��ȫ�����⻯����_______(����Բ����ƿ������������ȡ����)�С�
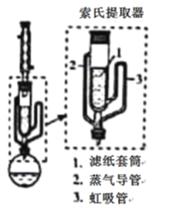
(6)����NaBH4�������ܼ������õķ�����_______��
(7)����Ч�⺬�����������������ԭ���Ļ�ԭ�����������ǣ�ÿ�˺��ԭ���Ļ�ԭ�����൱�ڶ��ٿ�H2�Ļ�ԭ������NaBH4����Ч�⺬��Ϊ_____________(������λС��)��
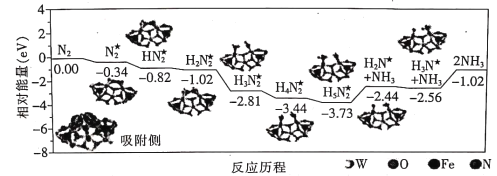
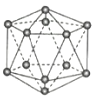
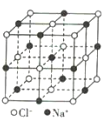
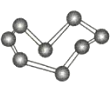
����Ŀ���۲�����ģ�Ͳ�����й���Ϣ���ж�����˵������ȷ���ǣ� ��
��������ÿ���ṹ��Ԫ����12��Bԭ�ӣ� | NaCl | S8 | HCN | |
�ṹģ��ʾ��ͼ |
|
|
|
|
��ע | �۵�2573K | ���� | ������CS2 | ���� |
A.����������ԭ�Ӿ��壬�ṹ��Ԫ�к���30��B-B������20����������
B.NaCl������ÿ��Na+��Χ�����������ȵ�Na+��6��
C.S8�����еĹ��ۼ�Ϊ�Ǽ��Լ�
D.HCN�������2��������2������