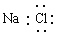
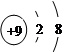��Ŀ����
����Hg��������Cr����Ӱ�컷����Ⱦ������ЧӦ�����ཡ�����ؽ���Ԫ�ء�
I�����㷺Ӧ���ڸ�����ҵ���Ŵ���¼����ȷ��档
��1����ҵ�����á����ڸ��������»�ԭ���̣�Cr2O3�����Ʊ������ʣ��÷�Ӧ�Ļ�ѧ����ʽΪ ��
II�������Ĺ�ҵ��ˮ�ᵼ�������ж���������+6�۸��ķ�ˮʱ�ɵõ������壨����ɿ�д��[Fe2+Fe3+(2-x) Cr3+x]O4����

��2���ӹ���FeSO4��Ŀ���� ��
��3����ƽ����ٵķ�Ӧ�����ӷ���ʽ Fe2++ Cr2O72��+ _____="=" Fe3++ Cr3++ H2O
�����������壨 [Fe2+Fe3+(2-x) Cr3+x]O4����X= _________________��
��4���±���ʵ�������Ķ�ij�����������������Ʒ��ÿ���������ȡ������6����Ʒ���������л��ʺ������IJⶨ��������±���
�ӱ�������Եó��Ľ����� ����һ�㼴�ɣ���
��5����ҵ���Ը���أ�K2CrO4��Ϊԭ�ϣ��绯ѧ���Ʊ��ظ���أ�װ����ͼ����ӦΪ��4K2CrO4+4H2O 2K2Cr2O7+4KOH+2H2��+O2����֪K2CrO4�������Һ�д���ƽ�⣺2CrO42��(��ɫ)+2H��
2K2Cr2O7+4KOH+2H2��+O2����֪K2CrO4�������Һ�д���ƽ�⣺2CrO42��(��ɫ)+2H�� Cr2O72��(��ɫ)+H2Oͨ�����������Һ�� ��Ϊ ��ԭ���� ��
Cr2O72��(��ɫ)+H2Oͨ�����������Һ�� ��Ϊ ��ԭ���� ��

I�����㷺Ӧ���ڸ�����ҵ���Ŵ���¼����ȷ��档
��1����ҵ�����á����ڸ��������»�ԭ���̣�Cr2O3�����Ʊ������ʣ��÷�Ӧ�Ļ�ѧ����ʽΪ ��
II�������Ĺ�ҵ��ˮ�ᵼ�������ж���������+6�۸��ķ�ˮʱ�ɵõ������壨����ɿ�д��[Fe2+Fe3+(2-x) Cr3+x]O4����

��2���ӹ���FeSO4��Ŀ���� ��
��3����ƽ����ٵķ�Ӧ�����ӷ���ʽ Fe2++ Cr2O72��+ _____="=" Fe3++ Cr3++ H2O
�����������壨 [Fe2+Fe3+(2-x) Cr3+x]O4����X= _________________��
��4���±���ʵ�������Ķ�ij�����������������Ʒ��ÿ���������ȡ������6����Ʒ���������л��ʺ������IJⶨ��������±���
| ��Ʒ��� | ȡ�����(m) | �л���(��10��2g) | �ܸ�(��10��6g) |
| ��ƷA-1 | 0.00 ~ 0.30 | 2.81 | 114 |
| ��ƷA-2 | 0.30 ~ 0.60 | 1.72 | 111 |
| ��ƷA-3 | 1.20 ~ 1.80 | 1.00 | 88 |
| ��ƷB-1 | 0.00 ~ 0.30 | 2.60 | 116 |
| ��ƷB-2 | 0.30 ~ 0.60 | 2.48 | 112 |
| ��ƷB-3 | 1.20 ~ 1.80 | 1.83 | 106 |
�ӱ�������Եó��Ľ����� ����һ�㼴�ɣ���
��5����ҵ���Ը���أ�K2CrO4��Ϊԭ�ϣ��绯ѧ���Ʊ��ظ���أ�װ����ͼ����ӦΪ��4K2CrO4+4H2O
 2K2Cr2O7+4KOH+2H2��+O2����֪K2CrO4�������Һ�д���ƽ�⣺2CrO42��(��ɫ)+2H��
2K2Cr2O7+4KOH+2H2��+O2����֪K2CrO4�������Һ�д���ƽ�⣺2CrO42��(��ɫ)+2H�� Cr2O72��(��ɫ)+H2Oͨ�����������Һ�� ��Ϊ ��ԭ���� ��
Cr2O72��(��ɫ)+H2Oͨ�����������Һ�� ��Ϊ ��ԭ���� ��
��15�֣���1��2Al + Cr2O3 2Cr+Al2O3��3�֣�
2Cr+Al2O3��3�֣�
��2����ԭCr2O72����Ϊ�������ṩFe2����2�֣�
��3��6��1��14H+��6��2��7��2�֣� 0.5��2�֣�
��4������ȡ����ȼ���л��ʵĺ������١��ܸ���Ҳ���٣������л��ʵ���Ⱦ��Դ����Ϊ����2�֣������������𰸾��ɣ�
��5����ɫ ��1�֣�����ɫ��1�֣�����������4OH����4e��==2H2O+O2����c(H+)����ʹ2CrO42��(��ɫ)+2H�� Cr2O72��(��ɫ)+H2Oƽ�������ƶ���2�֣�
Cr2O72��(��ɫ)+H2Oƽ�������ƶ���2�֣�
 2Cr+Al2O3��3�֣�
2Cr+Al2O3��3�֣���2����ԭCr2O72����Ϊ�������ṩFe2����2�֣�
��3��6��1��14H+��6��2��7��2�֣� 0.5��2�֣�
��4������ȡ����ȼ���л��ʵĺ������١��ܸ���Ҳ���٣������л��ʵ���Ⱦ��Դ����Ϊ����2�֣������������𰸾��ɣ�
��5����ɫ ��1�֣�����ɫ��1�֣�����������4OH����4e��==2H2O+O2����c(H+)����ʹ2CrO42��(��ɫ)+2H��
 Cr2O72��(��ɫ)+H2Oƽ�������ƶ���2�֣�
Cr2O72��(��ɫ)+H2Oƽ�������ƶ���2�֣������������1�������ȷ�Ӧ���ƿɵã�2Al + Cr2O3
 2Cr+Al2O3����2����������������Ŀ���ǽ�Cr2O72����ԭΪCr3+��Ϊ�������ṩFe2������3���ɵ��ӡ���ɡ�ԭ���غ��֪��6Fe2++Cr2O72��+14H+==6Fe3++2Cr3++7H2O����6Fe2++Cr2O72��+14H+==6Fe3++2Cr3++7H2O��֪��(2��x)/x=6/2����x=0.5����4��������֪������ȡ����ȼ���л��ʵĺ������١��ܸ���Ҳ���٣������л��ʵ���Ⱦ��Դ����Ϊ����5���������֪��������ӦʽΪ4OH����4e��==2H2O+O2������������c(OH��)��С��ʹ2CrO42��(��ɫ)+2H��
2Cr+Al2O3����2����������������Ŀ���ǽ�Cr2O72����ԭΪCr3+��Ϊ�������ṩFe2������3���ɵ��ӡ���ɡ�ԭ���غ��֪��6Fe2++Cr2O72��+14H+==6Fe3++2Cr3++7H2O����6Fe2++Cr2O72��+14H+==6Fe3++2Cr3++7H2O��֪��(2��x)/x=6/2����x=0.5����4��������֪������ȡ����ȼ���л��ʵĺ������١��ܸ���Ҳ���٣������л��ʵ���Ⱦ��Դ����Ϊ����5���������֪��������ӦʽΪ4OH����4e��==2H2O+O2������������c(OH��)��С��ʹ2CrO42��(��ɫ)+2H�� Cr2O72��(��ɫ)+H2O��ƽ�����ƣ���Һ�ɻ�ɫ��Ϊ��ɫ��
Cr2O72��(��ɫ)+H2O��ƽ�����ƣ���Һ�ɻ�ɫ��Ϊ��ɫ��
��ϰ��ϵ�д�
�����Ŀ
 MnCl2+Cl2��+2H2O
MnCl2+Cl2��+2H2O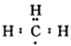 ���Ա�ʾ�������Dz����Ա�ʾCH+3
���Ա�ʾ�������Dz����Ա�ʾCH+3 ���Ա�ʾ
���Ա�ʾ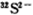 ���ֿ��Ա�ʾ34S2��
���ֿ��Ա�ʾ34S2�� ���Ա�ʾ������ӣ�Ҳ���Ա�ʾ���Ȼ�̼����
���Ա�ʾ������ӣ�Ҳ���Ա�ʾ���Ȼ�̼���� Si��2CO��������SiO2��
Si��2CO��������SiO2��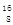 O��
O��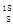 OΪ��ͬ�ĺ��أ��в�ͬ�Ļ�ѧ����
OΪ��ͬ�ĺ��أ��в�ͬ�Ļ�ѧ����