��Ŀ����
4��A��B��C��D��E���Ƕ�����Ԫ�أ�ԭ��������������B��Cͬ���ڣ�A��Dͬ���壮A��B���γ�����Һ̬��������ң�ԭ�Ӹ����ȷֱ�Ϊ2��1��1��1������������Ϣ�ش��������⣺
��1���ס����������к��зǼ��Թ��ۼ������ʵĵ���ʽ��
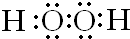 ��CԪ�������ڱ��е�λ���ǵڶ����ڡ��ڢ�A�壮
��CԪ�������ڱ��е�λ���ǵڶ����ڡ��ڢ�A�壮��2��C��D�������У��뾶��С����Na+�������ӷ��ţ���
��3����D�ĵ���Ͷ����У���D��ʧ������������Һ�м���E�ĵ��ʣ���ʱ������Ӧ�Ļ�ѧ����ʽ��2Al+2OH-+2H2O=2AlO2-+3H2����
��4��C��D��E��������ӻ�����DxEC6���侧�����������ھ����о��д����Ե���С�ظ���Ԫ���ṹ��ͼ��ʾ��������D+���á��ʾ��λ�������������е���������ڲ���������EC6x-���á��ʾ��λ�ڸ�������Ķ�������ģ��û�����Ļ�ѧʽ��Na3AlF6��
���� A��B��C��D��E���Ƕ���������Ԫ�أ�ԭ��������������A��B���γ�����Һ̬��������ң�ԭ�Ӹ����ȷֱ�Ϊ2��1��1��1����AΪH��BΪO��A��Dͬ���壬��DΪNa��B��Cͬ���ڣ���CΪF����ϣ�3��Al��Ӧ������������EΪAl��Ȼ������Ԫ�ؼ��䵥�ʡ�����������������
��� �⣺��1����ΪH2O��Ϊ�����Լ��ļ��Է��ӣ���ΪH2O2��Ϊ�����Լ����Ǽ��Լ��ļ��Է��ӣ����Է����������ǹ������⣬����ʽΪ��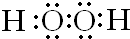 ��CΪF��λ�ڵڶ�����VIIA���ʴ�Ϊ��
��CΪF��λ�ڵڶ�����VIIA���ʴ�Ϊ��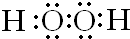 ���ڶ����ڡ��ڢ�A�壻
���ڶ����ڡ��ڢ�A�壻
��2�����Ӳ�����ͬ���˵����Խ��뾶ԽС��Na+��F-�ĵ��Ӳ�ṹ��ͬ�����������ӵİ뾶С���ʴ�Ϊ��Na+��
��3��Na��ˮ��Ӧ����NaOH��E��NaOH��Һ��Ӧ������������EΪAl�����ӷ�ӦΪ2Al+2OH-+2H2O=2AlO2-+3H2�����ʴ�Ϊ��2Al+2OH-+2H2O=2AlO2-+3H2����
��4���þ�����$\frac{1}{8}$�к��е������Ӹ���=1+4��$\frac{1}{8}$=$\frac{3}{2}$��������EC6x-����=4��$\frac{1}{8}$=$\frac{1}{2}$�������仯ѧʽ���������Ӹ���֮��Ϊ1��3����ѧʽΪNa3AlF6���ʴ�Ϊ��Na3AlF6��
���� ���⿼��λ�á��ṹ�����ʵĹ�ϵ��Ӧ�ã���ȷԪ�ص��ƶ��ǽ����Ĺؼ�����ϤԪ�����ڱ���Ԫ�������ɼ��ɽ���Ѷ��еȣ�

 ���Ͱ�ͨ��ĩ���ϵ�д�
���Ͱ�ͨ��ĩ���ϵ�д�| A�� | ͼ n��O2��=2molʱ��������C��O2���ܱ������еķ�Ӧ���� n��O2��=2molʱ��������C��O2���ܱ������еķ�Ӧ���� | |
| B�� | ͼ n��NaOH��=1mol ʱ��CO2��NaOH��Һ��Ӧ���ɵ��� n��NaOH��=1mol ʱ��CO2��NaOH��Һ��Ӧ���ɵ��� | |
| C�� |  n��HCl��=1mol ʱ��K2CO3��HCl��Һ�ڳ���������Ӧ���ɵ����� n��HCl��=1mol ʱ��K2CO3��HCl��Һ�ڳ���������Ӧ���ɵ����� | |
| D�� |  n��HNO3��=2mol ʱ��Fe��ϡ���ᷴӦ���ɵ����������ԭ����ΪNO�� n��HNO3��=2mol ʱ��Fe��ϡ���ᷴӦ���ɵ����������ԭ����ΪNO�� |
| A�� | ��Ԫ��ԭ�ӵ�������Ϊ112 | B�� | ��Ԫ��ԭ�ӵ�������Ϊ165 | ||
| C�� | ��Ԫ��ԭ�ӵĺ��������Ϊ112 | D�� | ��Ԫ��ԭ�ӵĺ˵����Ϊ277 |
| A�� | ��Ԫ��һ�����ٻ�����һ������������238��ͬλ�أ�XΪ���� | |
| B�� | �����������������˵�ͬλ�ص����ԭ�������������Ľ������ԭ��������XΪԭ�� | |
| C�� | ǭU�ı仯���ڻ�ѧ�仯��XΪ���� | |
| D�� | �˵ļ���ͬλ�صĻ�ѧ�������Բ�ͬ��XΪ���� |
| A�� | �٢ڢۢ� | B�� | �ܢڢۢ� | C�� | �ڢܢۢ� | D�� | �ܢ٢ڢ� |
| A�� | ���ά���ϳ���ά��������ά�����л��߷��ӻ����� | |
| B�� | �Ͻ���չ�˽������ϵ�ʹ�÷�Χ���Ͻ���Ҳ���ܺ��зǽ���Ԫ�� | |
| C�� | H2O2��һ����ɫ�����������������Ը�����ض�����O2 | |
| D�� | �ԡ��ع��͡����з�������Ƶ����͡�ú�ͣ��ﵽ���Ϊ����Ŀ�� |
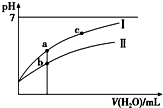 ��֪��25��ʱijЩ����ĵ���ƽ�ⳣ���������������ͼ���ʾ�����£�ϡ��CH3COOH��HClO�������ϡ��Һʱ����ҺpH���ˮ���ı仯������˵����ȷ���ǣ�������
��֪��25��ʱijЩ����ĵ���ƽ�ⳣ���������������ͼ���ʾ�����£�ϡ��CH3COOH��HClO�������ϡ��Һʱ����ҺpH���ˮ���ı仯������˵����ȷ���ǣ������� | CH3COOH | HClO | H2CO3 |
| Ka=1.8��10-5 | Ka=3.0��10-8 | Ka1=4.1��10-7 Ka2=5.6��10-11 |
| A�� | ��ͬŨ��CH3COONa��NaClO�Ļ��Һ�У�������Ũ�ȵĴ�С��ϵ��c��Na+����c��ClO-����c��CH3COO-����c��OH-����c��H+�� | |
| B�� | ��NaClO��Һ��ͨ������������̼�����ӷ���ʽΪ��2ClO-+CO2+H2O�T2HClO+CO32- | |
| C�� | ͼ����a��c���㴦����Һ��$\frac{c��{R}^{-}��}{c��HR��•c��O{H}^{-}��}$��ȣ�HR����CH3COOH��HClO�� | |
| D�� | ͼ����a�������Ũ�ȴ���b�������Ũ�� |