��Ŀ����
����Ŀ��25��ʱ��������ĵ���ƽ�ⳣ�����£�
��ѧʽ | CH3COOH | H2CO3 | HClO |
����ƽ�ⳣ�� | 1.8��10-5 | K14.3��10-7 K25.6��10-11 | 3.0��10-8 |
�ش��������⣺
(1)һ������£����¶�����ʱ��Ka__(����������������С������������)��
(2)�����������ӽ�����������ɴ�С��˳����__(�����)��
a.CO![]() b.ClO-c.CH3COO-d.HCO
b.ClO-c.CH3COO-d.HCO![]()
(3)���з�Ӧ���ܷ�������__(�����)
a.CO![]() +CH3COOH=CH3COO-+CO2��+H2O
+CH3COOH=CH3COO-+CO2��+H2O
b.ClO-+CH3COOH=CH3COO-+HClO
c.CO![]() +2HClO=CO2��+H2O+2ClO-
+2HClO=CO2��+H2O+2ClO-
d.2ClO-+CO2+H2O=CO![]() +2HClO
+2HClO
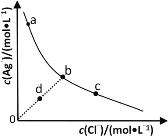
(4)�����Ϊ10mL��pH��Ϊ2�Ĵ�����Һ��HX��Һ�ֱ��ˮϡ����1000mL��ϡ������pH�仯��ͼ��ʾ��

��HX�ĵ���ƽ�ⳣ��__(������������������������С��������ͬ)����ĵ���ƽ�ⳣ����ϡ�ͺ�HX��Һ��ˮ���������c(H+)__������Һ��ˮ���������c(H+)��������___��
���𰸡����� a��b��d��c cd ���� ���� ϡ�ͺ������������Ũ�ȴ���HX����������ˮ����̶ȴ���HX
��������
�����¶ȴٽ�������ʵĵ��룻������Ӷ�Ӧ���������Խǿ��������ӽ�������ӵ�����Խ��������ǿ��ΪCH3COOH>H2CO3>HClO>HCO![]() ��ǿ���ܹ���ȡ�����ˮϡ�ʹٽ�������룬pH��ͬ�IJ�ͬ��ϡ����ͬ�ı�����pH�仯���������ǿ���仯С��������������������ˮ���룬���������ӻ��������������Ũ��Խ��������ˮ����̶�Խ��ˮϡ��HX��Һ��������Ũ�ȼ�С����ˮ�����Ƴ̶ȼ�С��
��ǿ���ܹ���ȡ�����ˮϡ�ʹٽ�������룬pH��ͬ�IJ�ͬ��ϡ����ͬ�ı�����pH�仯���������ǿ���仯С��������������������ˮ���룬���������ӻ��������������Ũ��Խ��������ˮ����̶�Խ��ˮϡ��HX��Һ��������Ũ�ȼ�С����ˮ�����Ƴ̶ȼ�С��
(1)�����¶��ܴٽ�������ʵĵ��룬���Ե��¶�����ʱ��Ka����
(2)����ƽ�ⳣ��Խ��Խ���룬��Һ������Ũ��Խ��������ǿ��Ϊ��CH3COOH��H2CO3��HClO��HCO![]() ��������Ӷ�Ӧ���������Խǿ��������ӽ�������ӵ�����Խ�������������ӽ�����ӵ������ɴ�С��˳���ǣ�CO
��������Ӷ�Ӧ���������Խǿ��������ӽ�������ӵ�����Խ�������������ӽ�����ӵ������ɴ�С��˳���ǣ�CO![]() ��ClO-��HCO
��ClO-��HCO![]() ��CH3COO-����a��b��d��c��
��CH3COO-����a��b��d��c��
(3)a.CO![]() +CH3COOH=CH3COO-+CO2��+H2O��̼�������С��CH3COOH������CH3COOH�ܹ���ȡ̼�ᣬ�÷�Ӧ�ܹ���������a���������⣻
+CH3COOH=CH3COO-+CO2��+H2O��̼�������С��CH3COOH������CH3COOH�ܹ���ȡ̼�ᣬ�÷�Ӧ�ܹ���������a���������⣻
b.ClO-+CH3COOH=CH3COO-+HClO��CH3COOH�����Դ���HClO��CH3COOH�ܹ���ȡHClO���÷�Ӧ�ܹ���������b���������⣻
c.CO![]() +HClO=CO2��+H2O+ClO-��HClO������С��̼�ᣬ�÷�Ӧ����������c�������⣻
+HClO=CO2��+H2O+ClO-��HClO������С��̼�ᣬ�÷�Ӧ����������c�������⣻
d.2ClO-+CO2+H2O=CO![]() +2HClO����������H2CO3��HClO��HCO
+2HClO����������H2CO3��HClO��HCO![]() ����̼�������������ӷ�Ӧֻ������̼��������ӣ���������CO
����̼�������������ӷ�Ӧֻ������̼��������ӣ���������CO![]() ���÷�Ӧ���ܷ�������d�������⣻
���÷�Ӧ���ܷ�������d�������⣻
����������������ǣ�cd��
(4)��ˮϡ�ʹٽ�������룬pH��ͬ�IJ�ͬ��ϡ����ͬ�ı�����pH�仯���������ǿ���仯С��������������������ˮ���룬���������ӻ��������������Ũ��Խ��������ˮ����̶�Խ����ͼ֪��pH��ͬ�Ĵ����HXϡ����ͬ�ı�����HX��pH�仯����HX�����Դ��ڴ��ᣬ����HX�ĵ���ƽ�ⳣ�����ڴ���ĵ���ƽ�ⳣ����ϡ�ͺ������������Ũ�ȴ���HX�����Դ�������ˮ����̶ȴ���HX����HX��Һ��ˮ���������c(H+)���ڴ�����Һˮ�������c(H+)�������ǣ�ϡ�ͺ������������Ũ�ȴ���HX����������ˮ����̶ȴ���HX��