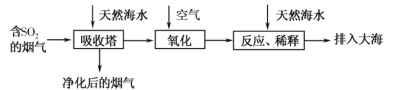
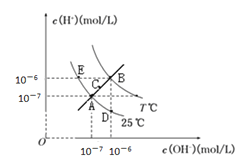
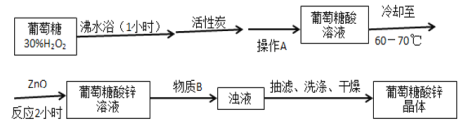
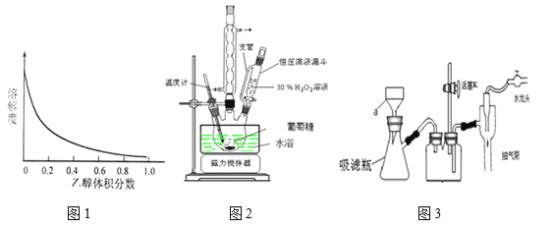
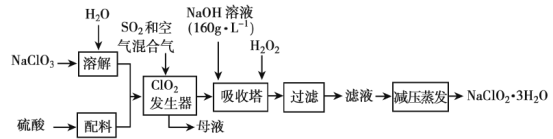
��Ŀ����
����Ŀ����ҵ��һ����CO��H2Ϊԭ�Ϻϳɼ״����÷�Ӧ���Ȼ�ѧ����ʽΪ��
CO(g)+ 2H2(g)![]() CH3OH(g) ��H
CH3OH(g) ��H
��1����֪CO(g)��H2(g)�ı�ȼ���ȷֱ�Ϊ ��H=��283kJmol-1�� ��H=��286kJmol-1����CH3OH(g)+3/2O2(g) ![]() CO2(g)+2H2O(l) ��H=��761kJmol-1����CO(g)+ 2H2(g)
CO2(g)+2H2O(l) ��H=��761kJmol-1����CO(g)+ 2H2(g)![]() CH3OH(g)����H= ___________��
CH3OH(g)����H= ___________��
��2��Ϊ�����CO��H2�Ʊ��״�����Ч�ʺͲ�������ҵ������ͨ����ȡ�Ĵ�ʩ��___________________________________________����д�����㣩
��3��ʵ����ģ����CO��H2��Ӧ���Ƽ״�����250 ���£���һ������CO��H2Ͷ��10 L���ܱ������У������ʵ����ʵ���Ũ�ȣ�mol��L-1���仯���±���ʾ��
2 min | 4 min | 6 min | |
CO | 0.07 | 0.05 | 0.05 |
H2 | x | 0.10 | 0.10 |
CH3OH | 0.03 | 0.05 | 0.05 |
��250 ��ʱ���÷�Ӧ��ƽ�ⳣ��K=___________��
����ͼ1�л�����Ӧ��ʼ����6 minʱH2�����ʵ����ı仯���ߣ���������ʵ������ꡣ_______
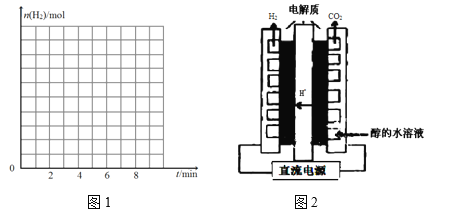
��4�����õ��״�ˮ��Һ�Ʊ����������ŵ������Ҫ�ĵ�ѹ�ͣ�װ����ͼ2��д�� �����缫��Ӧ����ʽ____________________________________________________�������ܷ�Ӧ��ѧ����ʽ__________________________________________��
���𰸡���94 kJmol-1 ԭ��ѭ��ʹ�ã�ʹ�ô�������ʱ���״�Һ�����룬���ƺ����¶ȣ���ѹ 100 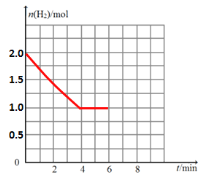 CH3OH��6e��+ H2O= CO2��+6 H+ CH3OH+ H2O
CH3OH��6e��+ H2O= CO2��+6 H+ CH3OH+ H2O![]() CO2��+3H2��
CO2��+3H2��
��������
��1����֪CO(g)��H2(g)�ı�ȼ���ȷֱ�Ϊ ��H=��283kJmol-1�� ��H=��286kJmol-1�����CO(g)+ 1/2O2(g)= CO2(g) ��H=��283kJmol-1����H2(g)+ 1/2O2(g)= H2O(l) ��H=��286kJmol-1��CH3OH(g)+3/2O2(g) ![]() CO2(g)+2H2O(l) ��H=��761kJmol-1����+��
CO2(g)+2H2O(l) ��H=��761kJmol-1����+��![]() 2-�ۣ�CO(g)+ 2H2(g)
2-�ۣ�CO(g)+ 2H2(g)![]() CH3OH(g)����H=��94 kJmol-1���𰸣���94 kJmol-1��
CH3OH(g)����H=��94 kJmol-1���𰸣���94 kJmol-1��
��2����CO(g)+ 2H2(g)![]() CH3OH(g)����H=��94 kJmol-1��֪���CO��H2�Ʊ��״�����Ч�ʺͲ�������Ҫ��ȡ�Ĵ�ʩ�ǣ�ԭ��ѭ��ʹ�ã�ʹ�ô�������ʱ���״�Һ�����룬���ƺ����¶ȣ���ѹ���𰸣�ԭ��ѭ��ʹ�ã�ʹ�ô�������ʱ���״�Һ�����룬���ƺ����¶ȣ���ѹ��
CH3OH(g)����H=��94 kJmol-1��֪���CO��H2�Ʊ��״�����Ч�ʺͲ�������Ҫ��ȡ�Ĵ�ʩ�ǣ�ԭ��ѭ��ʹ�ã�ʹ�ô�������ʱ���״�Һ�����룬���ƺ����¶ȣ���ѹ���𰸣�ԭ��ѭ��ʹ�ã�ʹ�ô�������ʱ���״�Һ�����룬���ƺ����¶ȣ���ѹ��
��3��������ͼ����֪4minʱCO(g)+ 2H2(g)![]() CH3OH(g)��Ӧ�ﵽƽ�⣬��ʱ�����ʵ�Ũ��Ϊ��c(CO)= 0.05 mol��L-1, c(H2)= 0.10 mol��L-1, c(CH3OH )=0.05 mol��L-1,���Ը÷�Ӧ��ƽ�ⳣ��K= c(CH3OH )/ c(CO)
CH3OH(g)��Ӧ�ﵽƽ�⣬��ʱ�����ʵ�Ũ��Ϊ��c(CO)= 0.05 mol��L-1, c(H2)= 0.10 mol��L-1, c(CH3OH )=0.05 mol��L-1,���Ը÷�Ӧ��ƽ�ⳣ��K= c(CH3OH )/ c(CO)![]() c2(H2)= 0.05/0.05
c2(H2)= 0.05/0.05![]() 0.10)2=100��
0.10)2=100��
�� CO(g) + 2H2(g)![]() CH3OH(g)
CH3OH(g)
��ʼŨ�ȣ�mol��L-1�� x y 0
�仯Ũ�ȣ�mol��L-1�� 0.05 0.10 0.05
ƽ��Ũ�ȣ�mol��L-1�� 0.05 0.10 0.05
����x=0.10 mol��L-1, y=0.20 mol��L-1��c(H2)��ʼ=0.20 mol��L-1��ƽ��Ũ��Ϊc(H2)ƽ��=0.10 mol��L-1����Ӧ��ʼ����6 minʱH2�����ʵ����ı仯����Ϊ
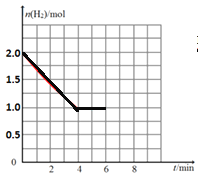 ��
��
��4�����õ��״�ˮ��Һ��װ��ͼ ��CH3OH![]() CO2֪CԪ�صĻ��ϼ����ߣ�ʧ���ӣ����������������缫��Ӧ����ʽΪ��CH3OH��6e��+ H2O= CO2��+6 H+ ��������H2O
CO2֪CԪ�صĻ��ϼ����ߣ�ʧ���ӣ����������������缫��Ӧ����ʽΪ��CH3OH��6e��+ H2O= CO2��+6 H+ ��������H2O![]() H2�����˻�ԭ��Ӧ�����Ե����ܷ�Ӧ��ѧ����ʽCH3OH+H2O
H2�����˻�ԭ��Ӧ�����Ե����ܷ�Ӧ��ѧ����ʽCH3OH+H2O![]() CO2��+3H2�����𰸣�CH3OH��6e��+ H2O= CO2��+6 H+��CH3OH+ H2O
CO2��+3H2�����𰸣�CH3OH��6e��+ H2O= CO2��+6 H+��CH3OH+ H2O![]() CO2��+3H2����
CO2��+3H2����
��

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�����Ŀ����������ʵ�����������ʵ�������ȷ����(����)
ѡ�� | ʵ�� | ���� | ʵ����� |
A | ��ij��Һ���ȵμ�����ϡ���ᣬ�ٵμ�����BaCl2��Һ | ���ְ�ɫ���� | ԭ��Һ�к���SO42-��SO32-��HSO3-�е�һ�ֻ��� |
B | ��װ��Fe(NO3)2��Һ���Թ��м���ϡ���� | �ڹܿڹ۲쵽����ɫ���� | HNO3�ֽ����NO2 |
C |
| ��������Ϊ��ɫ���ұ������Ϊ��ɫ | �����ԣ�Cl2>Br2>I2 |
D | SO2��SO3�������ͨ��Ba(NO3)2��Һ | ���ְ�ɫ���� | �õ��ij���ֻ��BaSO4 |
A. A B. B C. C D. D