��Ŀ����
20�� ��Ȼ�������������壬���ղ��ۺ�������������Ҫ�ľ��ü�ֵ�ͻ����������壮������ɾ���һϵ�з�Ӧ�Ƶ����
��Ȼ�������������壬���ղ��ۺ�������������Ҫ�ľ��ü�ֵ�ͻ����������壮������ɾ���һϵ�з�Ӧ�Ƶ����H2S$��_{��ȼ}^{O_{2}}$SO2$\stackrel{H_{2}O}{��}$H2SO3$\stackrel{����}{��}$H2SO4
��1�����ʷ�������ȫȼ��ʱ�ķ�Ӧ������ͨ��ʵ���ã���֪���������ȼ������586kJ/mol���������ʵ�ȼ������297��kJ•mol-1��д���������岻��ȫȼ�����ɹ������ʵ��Ȼ�ѧ����ʽH2S��g��+$\frac{1}{2}$O2��g��=S��s��+H2O ��l����H=-279kJ/mol��
��2����ҵ����������β������NaOH��Һ���գ�
������β����õ���Na2S��Һ�Լ��ԣ���ᡱ����������С�����
��������H2S��NaHS��Na2S��ص����ӷ���ʽ��ȷ���ǣ�����ĸ��ţ�AC��
A��H2S+OH-=HS-+H2O
B��HS-+H2O=H2S+OH-
C��HS-+H2O?S2-+H3O+
D��S2-+H2O?H2S+2OH-
��3����һ���¶��£�ij�����з���2H2S��g��?2H2��g��+S2��g���ķ�Ӧ�������Ӧʱ��ʱ�������ʵ�Ũ�ȣ�mol•L-1�����±������ݱ������ݻش����⣺
ʱ�� ���� | 0min | 20min | 60min | 90min | 120min |
| H2S | 0.006 | 0.005 | |||
| H2 | 0 | 0.002 | 0.004 | ||
| S2 | 0 | 0.002 | 0.0025 |
������¶��·�Ӧ�Ļ�ѧƽ�ⳣ��������д��������̣�K=0.0025 mol•L-1��
��4��������Ϊԭ�ϣ�ʹ�����ӹ������ʣ��ܴ���H+������ȼ�ϵ�أ�����ŵ����������������ѧʽS2������ȼ�ϵ�صĸ�����ӦʽΪ2H2S-4e-=S2+4H+��
��5��������ǿ�ᣬ��ͼ�л���������Һ������������Һ��Ӧ���̵������仯ʾ��ͼ��
���� ��1����֪S��ȼ����Ϊ297kJ•mol-1���Ȼ�ѧ����ʽΪ��S��s��+O2��g��=SO2��g����H=-297KJ/mol��
H2S��ȫȼ�յ��Ȼ�ѧ����ʽΪ��H2S��g��+$\frac{3}{2}$O2��g��=SO2��g��+H2O ��l����H=-586kJ/mol��
�ɸ�˹���ɢ�-�ٵõ�H2S����ȫȼ�����ɹ�̬���Һ̬ˮ��H2S��g��+$\frac{1}{2}$O2��g��=S��s��+H2O ��l����
��2����Na2SΪǿ�������Σ�������ˮ�⣻
��H2SΪ���ᣬNaHS��Na2SΪ�Σ�����ֲ����룬����ȫ���룬��ʽ������������ӷ�Ӧ�в��ܲ�֣�
��3������2H2S��g��?2H2��g��+S2��g���ķ�Ӧ��
��60 min��90 minH2S�ֽ���0.001 mol•L-1��ͬʱ��Ӧ����H20.001 mol•L-1��S20.0005 mol•L-1���������ڵ�H2��S2Ũ�ȷֱ�Ϊ0.005 mol•L-1��0.0025 mol•L-1����120 minʱ��S2Ũ����Ϊ0.0025 mol•L-1��
�ڽ��K=$\frac{{c}^{2}��{H}_{2}��c��{S}_{2}��}{{c}^{2}��{H}_{2}S��}$���㣻
��4������ŵ����������������ѧʽS2������������ʧȥ���ӣ�
��5��������Һ������������Һ��Ӧ��Ϊ�кͷ�Ӧ���ų�������
��� �⣺��1����֪S��ȼ����Ϊ297kJ•mol-1���Ȼ�ѧ����ʽΪ��S��s��+O2��g��=SO2��g����H=-297KJ/mol��
H2S��ȫȼ�յ��Ȼ�ѧ����ʽΪ��H2S��g��+$\frac{3}{2}$O2��g��=SO2��g��+H2O ��l����H=-586kJ/mol��
�ɸ�˹���ɢ�-�ٵõ�H2S����ȫȼ�����ɹ�̬���Һ̬ˮ��H2S��g��+$\frac{1}{2}$O2��g��=S��s��+H2O ��l����H=-279kJ/mol��
�ʴ�Ϊ��H2S��g��+$\frac{1}{2}$O2��g��=S��s��+H2O ��l����H=-279kJ/mol��
��2����Na2SΪǿ�������Σ�������ˮ�⣬����Һ�Լ��ԣ��ʴ�Ϊ���
��A��H2S+OH-=HS-+H2O��Ϊ����к͵����ӷ�Ӧ����A��ȷ��
B��HS-+H2O=H2S+OH-��ˮ��Ϊ���淴Ӧ��Ӧ����=����Ϊ��?������B����
C��HS-+H2O?S2-+H3O+��Ϊ��������ӷ�Ӧ����C��ȷ��
D��S2-+H2O?H2S+2OH-��������ˮ��ֲ����У��Ե�һ��Ϊ������D����
�ʴ�Ϊ��AC��
��3������2H2S��g��?2H2��g��+S2��g���ķ�Ӧ��
��60 min��90 minH2S�ֽ���0.001 mol•L-1��ͬʱ��Ӧ����H20.001 mol•L-1��S20.0005 mol•L-1���������ڵ�H2��S2Ũ�ȷֱ�Ϊ0.005 mol•L-1��0.0025 mol•L-1����120 minʱ��S2Ũ����Ϊ0.0025 mol•L-1����90 minʱ��Ӧ�Ѵ�ƽ��״̬�������淴Ӧ������ȣ�90minʱ��Ӧ����v������=v���棩���ʴ�Ϊ��=��
��$K=\frac{{{c^2}��{H_2}��•c��{S_2}��}}{{{c^2}��{{H_2}S}��}}=\frac{{{{��0.005\;mol•{L^{-1}}��}^2}��0.0025\;mol•{L^{-1}}}}{{{{��0.005\;mol•{L^{-1}}��}^2}}}=0.0025\;mol•{L^{-1}}$���ʴ�Ϊ��0.0025 mol•L-1��
��4������ŵ����������������ѧʽS2������������ʧȥ���ӣ���ȼ�ϵ�صĸ�����ӦʽΪ2H2S-4e-=S2+4H+���ʴ�Ϊ��2H2S-4e-=S2+4H+��
��5��������Һ������������Һ��Ӧ��Ϊ�кͷ�Ӧ���ų���������Ӧ���̵������仯ʾ��ͼΪ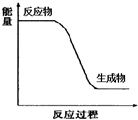 ���ʴ�Ϊ��
���ʴ�Ϊ�� ��
��
���� ���⿼����ۺϣ�Ϊ��Ƶ���㣬�漰�Ȼ�ѧ��Ӧ������ˮ�⡢��ѧƽ��ļ��㼰ԭ��صȣ����ػ�ѧ��Ӧԭ����Ӧ�������Ŀ��飬��Ŀ�Ѷ��еȣ�

 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�| A�� | 0.1mol/LNa2CO3��Һ�У�2c��Na+��=c��HCO3-��+c��H2CO3��+c��C032-�� | |
| B�� | ��0.1mol/L�Ȼ����Һ�еμ�Ũ���������c��NH4+��=c��H+�� | |
| C�� | pH=4�Ĵ�����pH=10��NaOH��Һ�������Ϻ�pH��7 | |
| D�� | ��0.2mol/LNaA��Һ��ijŨ�ȵ����������������������Һ�У�c��Na+��=c��A-��+c��Cl-��=0.2mol/L |
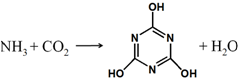 CO2����Դ�������ǽ������ЧӦ����Ҫ;������ͼ����һ����������NH3����CO2������Ҫ������Ʒ��������ķ�Ӧ�������й����������˵����ȷ���ǣ�������
CO2����Դ�������ǽ������ЧӦ����Ҫ;������ͼ����һ����������NH3����CO2������Ҫ������Ʒ��������ķ�Ӧ�������й����������˵����ȷ���ǣ�������| A�� | ����ʽΪC3H6N3O3 | B�� | �����мȺ��ЦҼ��ֺ��Цм� | ||
| C�� | �����мȺ����Լ����ֺ��Ǽ��Լ� | D�� | ���ɸ����ʵ�������ӦΪ�кͷ�Ӧ |
| A�� | ԭ�Ӱ뾶��A��D��C��B | |
| B�� | ����⻯����ȶ��ԣ�D��C | |
| C�� | A��C�γɵĻ���������ˮ������Һ�Լ��� | |
| D�� | B��D�γɵĻ���������ˮ������Һ�Լ��� |
| A�� | ���һ�����Ǹ߷��ӻ������ˮ�������ͬ | |
| B�� | �ױ���ʹ���Ը��������Һ��ɫ��֤���ױ������д��ڵ�˫������Ľṹ | |
| C�� | ������ʳ��ƾ������ɵ��ۡ������ǡ��Ҵ��Ļ�ѧ�仯���� | |
| D�� | �״����Ҷ�����HOCH2CH2OH����Ϊͬϵ�� |
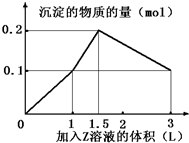 ij�����Һ�У�������X��Y��0.1mol�������еμ�0.1mol/L��Z��Һ�����ó��������ʵ�����ͼ�������������X��Y��Z�ֱ��ǣ�������
ij�����Һ�У�������X��Y��0.1mol�������еμ�0.1mol/L��Z��Һ�����ó��������ʵ�����ͼ�������������X��Y��Z�ֱ��ǣ�������| A�� | �Ȼ������Ȼ������������� | B�� | �Ȼ������Ȼ�þ���������� | ||
| C�� | ƫ�����ơ��Ȼ��������� | D�� | ƫ�����ơ��������������� |
| A�� | ��������Һ�м����������ռ���Һ��Al3++3OH-=Al��OH��3�� | |
| B�� | �ں��е����ʵ�����Fe��NO3��2��KI��ɵĻ����Һ�е���ϡ���3Fe2++4H++NO3-=3Fe3++NO��+2H2O | |
| C�� | ̼�������Һ�еμ�������NaOH��Һ��HCO3-+OH-=CO32-+H2O | |
| D�� | ������Һ�ʼ��Ե�ԭ��CO32-+H2O?HCO3-+OH- |
| A�� | ��0.1mol•L-1NaHCO3��Һ�У�c��Na+����c��HCO3-����c��CO32-����c��H2CO3�� | |
| B�� | ��0.1mol•L-1Na2CO3��Һ�У�c��OH-��+c��H+��=c��HCO3-��+2c��H2CO3�� | |
| C�� | ��0.2mol•L-1NaHCO3��Һ�м�������0.1mol•L-1NaOH��Һ��c��CO32-����c��HCO3-����c��OH-����c��H+�� | |
| D�� | �����£�CH2COONa��CH3COOH�����Һ[pH=7��c��Na+��=0.1mol•L-1]��c��Na+��=c��CH3COO-����c��CH3COOH����c��H+��=c��OH-�� |