��Ŀ����
19��Na��Fe��C��O��Si��Cl����ѧ��ѧ�г���������Ԫ�أ���1��Siλ��Ԫ�����ڱ��������ڵڢ�A�壻Fe�Ļ�̬ԭ����Χ���Ӳ��Ų�ʽΪ3d64s2��
��2���á�����������Ԫ�ط�����գ�
| C��O��Si��һ������ | ���Ӱ뾶Cl-��Na+��O2- | �۵� | �縺�� |
| Si��C��O | Cl-��O2-��Na+ | CO2��SiO2 | Cl��Si |
��4��FeO42- ����ǿ�����ԣ�����������Һ�м���ϡ���ᣬ��Һ��Ϊ��ɫ��������ɫ����������÷�Ӧ�����ӷ���ʽΪ4 FeO42-+20 H+=4Fe3++3O2��+10H2O��
���� ��1������Ԫ��������=���Ӳ���������������=������������FeΪ26��Ԫ�أ����ݺ�������Ų�������д��Χ�����Ų�ʽ��
��2��ͬ������ԭ����������Ԫ�ص�һ�����ܳ��������ƣ�ͬ�������϶��µ�һ�����ܼ�С��
���Ӳ�ṹ��ͬ�����ӣ��˵����Խ�����Ӱ뾶ԽС�����Ӳ�Խ�����Ӱ뾶Խ��
�۷е�ߵ�һ��Ϊ��ԭ�Ӿ��壾���Ӿ��壾���Ӿ��壻
ͬ������ԭ���������縺������
��3��ע�����ʾۼ�״̬���H��д�Ȼ�ѧ����ʽ��
��4��FeO42- ����ǿ�����ԣ�����������Һ�м���ϡ���ᣬ��Һ��Ϊ��ɫ��˵����Fe3+���ɣ�������ɫ������������ݵ���ת���غ㣬ֻ��ΪOԪ�ر��������ʻ�����O2����ƽ��д���ӷ���ʽ��
��� �⣺��1��SiԪ��ԭ�Ӻ�����3�����Ӳ㣬����������Ϊ4���������ڱ��е������ڢ�A�壻FeΪ26��Ԫ�أ���������Ų�Ϊ1s22s22p63s23p63d64s2����Χ�����Ų�ʽΪ3d64s2��
�ʴ�Ϊ��������A��3d64s2��
��2��ͬ������ԭ����������Ԫ�ص�һ�����ܳ��������ƣ�ͬ�������϶��µ�һ�����ܼ�С���ʵ�һ�����ܣ�Si��C��O��
���Ӳ�ṹ��ͬ�����ӣ��˵����Խ�����Ӱ뾶ԽС�����Ӳ�Խ�����Ӱ뾶Խ�����Ӱ뾶��Cl-��O2-��Na+��
������̼Ϊ���Ӿ��壬������������ԭ�Ӿ��壬���۵㣺CO2��SiO2��
ͬ������ԭ���������縺�����ʵ縺�ԣ�Cl��Si��
�ʴ�Ϊ��Si��C��O��Cl-��O2-��Na+��CO2��SiO2��Cl��Si��
��3����֪�״���ȼ����Ϊ726.5KJ/mol���״�ȼ�յ��Ȼ�ѧ����ʽΪ��CH3OH��l��+$\frac{3}{2}$O2��g��=CO2��g��+H2O��l����H=-726.5KJ/mol��
�ʴ�Ϊ��CH3OH��l��+$\frac{3}{2}$O2��g��=CO2��g��+H2O��l����H=-726.5KJ/mol��
��4��FeO42- ����ǿ�����ԣ�����������Һ�м���ϡ���ᣬ��Һ��Ϊ��ɫ��˵����Fe3+���ɣ�������ɫ������������ݵ���ת���غ㣬ֻ��ΪOԪ�ر��������ʻ�����O2����Ӧ���ӷ���ʽΪ��4 FeO42-+20 H+=4Fe3++3O2��+10H2O��
�ʴ�Ϊ��4 FeO42-+20 H+=4Fe3++3O2��+10H2O��
���� ���⿼��ṹ����λ�ù�ϵӦ�á���������Ų������뾶�Ƚϡ��������������ʡ��Ȼ�ѧ����ʽ�����ӷ���ʽ�ȣ��ѵ��ǣ�4�������ӷ���ʽ����д��ע��Ի���֪ʶ���������գ�

 ���㼤�������100�ִ��Ծ�ϵ�д�
���㼤�������100�ִ��Ծ�ϵ�д�| A�� | CH3CH2OH$��_{170��}^{Ũ����}$CH2�TCH2��+H2O | |
| B�� | CH3CHBrCH3+NaOH$��_{��}^{�Ҵ�}$CH3CH�TCH2��+NaBr+H2O | |
| C�� | 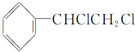 +2NaOH$��_{��}^{�Ҵ�}$ +2NaOH$��_{��}^{�Ҵ�}$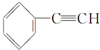 +2NaCl+2H2O +2NaCl+2H2O | |
| D�� | 2CH3OH$��_{��}^{Ũ����}$CH3-O-CH3+H2O |
| A�� | ��ͬ��ԭ�� | B�� | ͬһԪ�صIJ�ͬԭ�ӻ����� | ||
| C�� | ��ͬԪ�ص����� | D�� | ��ͬ��Ԫ�� |
| A�� | B�� |
| ��H-I�����ܴ���H-Cl���� ��H-I������С��H-Cl���� ��HI���Ӽ�����������HCl���Ӽ������� ����HI���Ӽ�������С��HCl���Ӽ������� | a��HI��HCl�ȶ� b��HCl��HI�ȶ� c��HI�е��HCl�� d��HI�е��HCl�� |
�٢�a���ڢ�b���ۢ�c���ܢ�d��
| A�� | �٢� | B�� | �ڢ� | C�� | �٢� | D�� | �ڢ� |
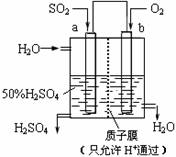
| A�� | a������b���� | |
| B�� | ����������a�缫�������� | |
| C�� | ���Ӵ�b����a���ƶ� | |
| D�� | ������ӦʽΪ��SO2+2H2O-2e-=SO42-+4H+ |
| A�� | ��ϩ | B�� | ���� | C�� | ��ϩ | D�� | ���� |
| A�� | ǿ������ | B�� | ��ˮ�� | C�� | ���� | D�� | ��ˮ�� |

��1����ɫ��������BaSO4��
��2����д��ʵ���������������A��B�����ӷ���ʽCO32-+2H+=H2O+CO2����NH+4+OH-=NH3��+H2O��
��3��ͨ������ʵ�飬��ȷ��X��Һ��һ�����ڵ�������NH+4��CO32-��SO42-��Ҫȷ�����ܴ��ڵ����ӣ��貹�ӵ�ʵ����K+��
��A��B��C����ǿ����ʣ�������ˮ�е�������������±���ʾ��
| ������ | Na+��K+��Cu2+ |
| ������ | SO${\;}_{4}^{2-}$��OH- |
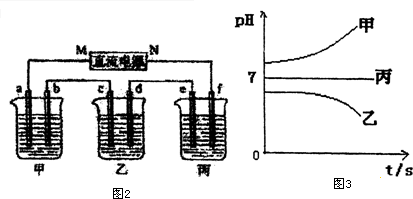
��ͨ��Դ������һ��ʱ��������c�缫����������16g�������¸��ձ�����Һ��pH����ʱ��t�Ĺ�ϵͼ��ͼ3���ݴ˻ش��������⣺
��1��MΪ��Դ�ĸ�������д�������������缫b�Ϸ����ĵ缫��ӦΪ4OH--4e-=O2��+2H2O��
��2������缫e�����ɵ������ڱ�״���µ����5.6L��
��3��д�����ձ��ĵ��ط�Ӧ2CuSO4+2H2O$\frac{\underline{\;���\;}}{\;}$2Cu+O2��+2H2SO4��
��4�������������B��Һ�еĽ�������ȫ����������ʱ����ܷ�������У�Ϊʲô��
��5��������һ��ʱ��������c�缫����������16g��Ҫʹ���ָ���ԭ����״̬�������Ǽ���4.5gˮ��