��Ŀ����
��14�֣�������أ�K2FeO4����һ�ּ�������������
������ɱ���������ȥ�ǡ���ɫ������Ϊһ�����
�͡���Ч����ɫ�����Ķ��ˮ����������ʮ����
�����ҹ��Ը������������ˮ�����е�Ӧ�õ��о�
Ҳ�������룬��ȡ�ÿ�ϲ�ɹ����Ƚ�������Ʊ���
���Ǵ�������������������KOH��Һ��ͨ������
Cl2�Ʊ�������ر�����Һ���ٷִμ���KOH���壬
�õ��������ǿ���Ա�����Һ�������������Σ��ϳɸ�����ء�
��1����������ǿ�����Һ�м����������η�����Ӧ�����ӷ���ʽ��
��Fe3++3OH-=Fe��OH��3���� ��
��2�������������ˮ���ͷŴ�����ԭ�������Ӷ��dz���Ч��ɱ��ˮ�еIJ����Ͳ��������ͬʱ����������ԭ������̬��Fe��OH��3������һ��Ʒ�������������������ܸ�Ч�س�ȥˮ�е�ϸ�����������K2Fe2O4��Һ��pH=4.74����Һ�У����Ƴ�c(FeO2-4) =1.0mmol��L-1�������������ֱ�����20�桢30�桢40���60��ĺ���ˮԡ�У��ⶨc(FeO2-4)�ı仯���������ͼ�����������ˮ��Ӧ�����ӷ�Ӧ����ʽΪ ���÷�Ӧ�ġ�H 0���>����<����=������
��3���������λ���һ��������ܵ�صIJ��ϣ��������ɵĵ�������ߣ��ŵ�������ܳ�ʱ�䱣���ȶ��ķŵ��ѹ��������ص��ܷ�ӦΪ��
3Zn+2K2FeO4+8H2O 3Zn��OH��3+2Fe��OH��3+4KOH
�õ�طŵ�ʱ�ĸ�����ӦʽΪ �������·��5.418��1022������ͨ������������ g������ز��뷴Ӧ��
��4���ⶨijK2FeO4��ҺŨ�ȵ�ʵ�鲽�����£�
����1��ȷ��ȡV mL K2FeO4��Һ���뵽��ƿ��
����2����ǿ������Һ�У��ù���CrO-2��FeO2-4��Ӧ����Fe��OH��3��CrO2-4
����3��������ϡ���ᣬʹCrO2-4ת��ΪCr2O2-2��CrO-2ת��ΪCr3+��Fe��OH��3ת��ΪFe2+
����4�������������������ָʾ������c mol��L-1��NH4��2Fe��SO4��2����Һ�ζ����յ㣬���ģ�NH4��2Fe��SO4��2��ҺV1mL��
�ٵζ�ʱ������Ӧ�����ӷ���ʽΪ ��
��ԭ��Һ��K2FeO4��Ũ��Ϊ ���ú���ĸ�Ĵ���ʽ��ʾ����
������ɱ���������ȥ�ǡ���ɫ������Ϊһ�����
�͡���Ч����ɫ�����Ķ��ˮ����������ʮ����
�����ҹ��Ը������������ˮ�����е�Ӧ�õ��о�
Ҳ�������룬��ȡ�ÿ�ϲ�ɹ����Ƚ�������Ʊ���
���Ǵ�������������������KOH��Һ��ͨ������
Cl2�Ʊ�������ر�����Һ���ٷִμ���KOH���壬
�õ��������ǿ���Ա�����Һ�������������Σ��ϳɸ�����ء�
��1����������ǿ�����Һ�м����������η�����Ӧ�����ӷ���ʽ��
��Fe3++3OH-=Fe��OH��3���� ��
��2�������������ˮ���ͷŴ�����ԭ�������Ӷ��dz���Ч��ɱ��ˮ�еIJ����Ͳ��������ͬʱ����������ԭ������̬��Fe��OH��3������һ��Ʒ�������������������ܸ�Ч�س�ȥˮ�е�ϸ�����������K2Fe2O4��Һ��pH=4.74����Һ�У����Ƴ�c(FeO2-4) =1.0mmol��L-1�������������ֱ�����20�桢30�桢40���60��ĺ���ˮԡ�У��ⶨc(FeO2-4)�ı仯���������ͼ�����������ˮ��Ӧ�����ӷ�Ӧ����ʽΪ ���÷�Ӧ�ġ�H 0���>����<����=������
��3���������λ���һ��������ܵ�صIJ��ϣ��������ɵĵ�������ߣ��ŵ�������ܳ�ʱ�䱣���ȶ��ķŵ��ѹ��������ص��ܷ�ӦΪ��
 |
3Zn+2K2FeO4+8H2O 3Zn��OH��3+2Fe��OH��3+4KOH
�õ�طŵ�ʱ�ĸ�����ӦʽΪ �������·��5.418��1022������ͨ������������ g������ز��뷴Ӧ��
��4���ⶨijK2FeO4��ҺŨ�ȵ�ʵ�鲽�����£�
����1��ȷ��ȡV mL K2FeO4��Һ���뵽��ƿ��
����2����ǿ������Һ�У��ù���CrO-2��FeO2-4��Ӧ����Fe��OH��3��CrO2-4
����3��������ϡ���ᣬʹCrO2-4ת��ΪCr2O2-2��CrO-2ת��ΪCr3+��Fe��OH��3ת��ΪFe2+
����4�������������������ָʾ������c mol��L-1��NH4��2Fe��SO4��2����Һ�ζ����յ㣬���ģ�NH4��2Fe��SO4��2��ҺV1mL��
�ٵζ�ʱ������Ӧ�����ӷ���ʽΪ ��
��ԭ��Һ��K2FeO4��Ũ��Ϊ ���ú���ĸ�Ĵ���ʽ��ʾ����
��14�֣�
��1��2Fe��OH��3+3ClO-+10OH-=2FeO2-4+3Cl-+5H2O ��������2��
 ��2��4FeO2-4+10H2O 4Fe��OH��3+8OH-+3O2����> ��������2��
��2��4FeO2-4+10H2O 4Fe��OH��3+8OH-+3O2����> ��������2��
��3��Zn+2OH��2e-=Zn��OH����5.94 ��������2��
��4��6Fe2++Cr2O2-7+14H+=6Fe3++2Cr3++7H2O��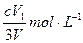 ��������2��
��������2��
��1��2Fe��OH��3+3ClO-+10OH-=2FeO2-4+3Cl-+5H2O ��������2��
 ��2��4FeO2-4+10H2O 4Fe��OH��3+8OH-+3O2����> ��������2��
��2��4FeO2-4+10H2O 4Fe��OH��3+8OH-+3O2����> ��������2����3��Zn+2OH��2e-=Zn��OH����5.94 ��������2��
��4��6Fe2++Cr2O2-7+14H+=6Fe3++2Cr3++7H2O��
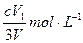 ��������2��
��������2����

��ϰ��ϵ�д�
 �п�������㾫��ϵ�д�
�п�������㾫��ϵ�д�
�����Ŀ
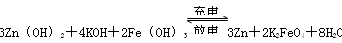 �����ж���ȷ����
�����ж���ȷ���� Ϊ����ʵ������ֻ��Ŀɳ��ĸ�Чȼ�ϵ�أ���һ�ε������ʹ��һ���¡���ͼ��һ���绯ѧ���̵�ʾ��ͼ����֪�׳ص��ܷ�ӦʽΪ��2CH3OH+3O2+4KOH
Ϊ����ʵ������ֻ��Ŀɳ��ĸ�Чȼ�ϵ�أ���һ�ε������ʹ��һ���¡���ͼ��һ���绯ѧ���̵�ʾ��ͼ����֪�׳ص��ܷ�ӦʽΪ��2CH3OH+3O2+4KOH 2K2CO3+6H2O
2K2CO3+6H2O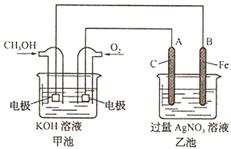
 _________L����״���£���
_________L����״���£���
 Fe��OH��2 + Ni��OH��2���ڴ�����������˵����
Fe��OH��2 + Ni��OH��2���ڴ�����������˵���� Ϊ������
������ ;
;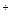 +2e-;
+2e-;
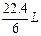
 Li2S2O4������˵����ȷ���ǣ� ��
Li2S2O4������˵����ȷ���ǣ� ��