��Ŀ����
2�� ��֪H2C2O4Ϊ��Ԫ��ǿ�ᣮij��ѧ��ȤС��Ϊ�ⶨ��Na2SO4��NaHC2O4��H2C2O4•2H2O�������и����ʵ���������������������ʵ�飺
��֪H2C2O4Ϊ��Ԫ��ǿ�ᣮij��ѧ��ȤС��Ϊ�ⶨ��Na2SO4��NaHC2O4��H2C2O4•2H2O�������и����ʵ���������������������ʵ�飺�ٳ�ȡ10.0g��������ˮ�ܽ⣬���250mL������Һ
������ʽ�ζ��ֱܷ���ȡ25.00mL������Һ��������ƿ��
�۵�һ����Һ�м�2��3��ָʾ������0.2500mol•L-1NaOH��Һ�ζ�������NaOH��Һ20.00mL
�ܵڶ�����Һ��0.1000mol•L-1�����Ը��������Һ�ζ������ĸ��������Һ16.00mL
�ش��������⣺
��1��д��NaHC2O4��Һ��NaOH��Һ��Ӧ�����ӷ���ʽHC2O4-+OH-=H2O+C2O42-��
��2�����������������Һʱ����Ҫ�IJ����������ձ�������������Ͳ����ͷ�ιܡ�250mL����ƿ��
��3����������ζ��յ�ʱ��Һ��pH=8.3����ѡ���ָʾ��Ϊ��̪������ij���̶ֿ�ģ�������50mL�ζ��ܽ���ʵ�飬���ζ����е�Һ�洦����ͼ��ʾ�Ŀ̶ȴ��������Һ������D ������ţ���
a������23.60mL b������27.60mL c����23.60mL d������27.60mL
��4���ζ�ʱ�ߵα�ҡ����ƿ���۾�Ӧ�۲���ƿ����Һ��ɫ�ı仯��
��5����ɲ���ƽ�������ӷ���ʽ��
5C2O${\;}_{4}^{2-}$+2MnO${\;}_{4}^{-}$+6H+=10CO2��+2Mn2++8H2O��
��6��������У����������ҺӦװ����ʽ �ζ�����ò��жϵζ��յ�ķ�������Һ����ɫ��Ϊdz��ɫ���ڰ�����ڲ���ɫ��
��7��������Na2SO4����������Ϊ53.8%������3λ��Ч���֣���
���� ��1��NaHC2O4��Һ��NaOH��Һ��Ӧ����Na2C2O4��ˮ��
��2������������Һʱ����Ҫ�IJ����������ձ�������������Ͳ����ͷ�ιܡ�250mL����ƿ��
��3����������ζ��յ�ʱ��Һ��pH=8.3����Ӧѡ�����ָʾ�����ζ���0�̶������棬ÿһ��С�̶�Ϊ0.1mL���ݴ˴��⣻
��4�����ݵζ�����Ҫ���۾�Ӧ�۲���ƿ����Һ��ɫ�ı仯��
��5����Ӧ��̼��+3����Ϊ+4�ۣ��̴�+7�۽�Ϊ+2�ۣ����ݻ�����������Ԫ���غ�͵���غ���ƽ���ӷ���ʽ��
��6��������У����������Һ��ǿ�����ԣ��������齺�ܣ��ζ��յ�ʱ��Һ����ɫ���dz��ɫ��
��7������Ʒ��NaHC2O4Ϊxmol��H2C2O4•2H2OΪymol�����ݵ�һ����Һ�м�2��3��ָʾ������0.2500mol•L-1 NaOH��Һ�ζ�������NaOH��Һ20.00mL�ɵ�x+2y=0.2500��0.02mol=0.005mol�����ݵڶ�����Һ��0.1000mol•L-1�����Ը��������Һ�ζ������ĸ��������Һ16.00mL����ϵ��ӵ�ʧ�غ㣬�ɵ�x+y=$\frac{5}{2}$��0.1000��0.016mol=0.004mol���ݴ˼����NaHC2O4��H2C2O4•2H2O����������ȷ��������Na2SO4������������
��� �⣺��1��NaHC2O4��Һ��NaOH��Һ��Ӧ����Na2C2O4��ˮ����Ӧ�����ӷ���ʽΪHC2O4-+OH-=H2O+C2O42-��
�ʴ�Ϊ��HC2O4-+OH-=H2O+C2O42-��
��2������������Һʱ����Ҫ�IJ����������ձ�������������Ͳ����ͷ�ιܡ�250mL����ƿ��
�ʴ�Ϊ����ͷ�ιܡ�250mL����ƿ��
��3����������ζ��յ�ʱ��Һ��pH=8.3����Ӧѡ�����ָʾ��Ϊ��̪���ζ���0�̶������棬ÿһ��С�̶�Ϊ0.1mL�����Ը���ͼ�ϵĿ̶ȹ���Һ������ӦΪ����27.60mL����ѡD��
�ʴ�Ϊ����̪��D��
��4�����ݵζ�����Ҫ���۾�Ӧ�۲���ƿ����Һ��ɫ�ı仯��
�ʴ�Ϊ����ƿ����Һ��ɫ�ı仯��
��5����Ӧ��̼��+3����Ϊ+4�ۣ��̴�+7�۽�Ϊ+2�ۣ����ݻ�����������Ԫ���غ�͵���غ��֪���ӷ���ʽΪ5H2C2O4+2MnO4-+6H+=10CO2��+2Mn2++8H2O��
�ʴ�Ϊ��5��2��6��10��2��8H2O��
��6��������У����������Һ��ǿ�����ԣ��������齺�ܣ����Ը��������ҺӦװ����ʽ�ζ�����жϵζ��յ�ķ�������Һ����ɫ��Ϊdz��ɫ���ڰ�����ڲ���ɫ��
�ʴ�Ϊ����ʽ����Һ����ɫ��Ϊdz��ɫ���ڰ�����ڲ���ɫ��
��7������Ʒ��NaHC2O4Ϊxmol��H2C2O4•2H2OΪymol�����ݵ�һ����Һ�м�2��3��ָʾ������0.2500mol•L-1 NaOH��Һ�ζ�������NaOH��Һ20.00mL�ɵ�x+2y=0.2500��0.02mol=0.005mol�����ݵڶ�����Һ��0.1000mol•L-1�����Ը��������Һ�ζ������ĸ��������Һ16.00mL����ϵ��ӵ�ʧ�غ㣬�ɵ�x+y=$\frac{5}{2}$��0.1000��0.016mol=0.004mol������$\left\{\begin{array}{l}{x+2y=0.005}\\{x+y=0.004}\end{array}\right.$�����x=0.003��y=0.001������10.0g�����к���NaHC2O4������Ϊ$\frac{250}{25}$��112��0.003g=3.36g��H2C2O4•2H2O������Ϊ$\frac{250}{25}$��126��0.001g=1.26g����������Na2SO4����������Ϊ$\frac{10-1.26-3.36}{10}$��100%=53.8%��
�ʴ�Ϊ��53.8%��
���� ���⿼��������ԭ�ζ�ԭ����Ӧ�á�̽��Ӱ�����ʵ����أ��Ѷ��еȣ�����ʵ��ԭ���ǽ���Ĺؼ����Ƕ�֪ʶ���ۺ����ã���Ҫѧ���߱���ʵ�Ļ���֪ʶ������֪ʶ�������⡢��������������

| �� | �� | |
| �� | ��Fe����ϡ������ | ��Fe����ϡ������ |
| �� | Ca��HCO3��2��Һ�м���������NaOH��Һ | Ca��OH��2��Һ�м���������NaHCO3��Һ |
| �� | ��0.1mol Cl2ͨ�뺬0.3mol FeBr2����Һ�� | ��0.3mol Cl2ͨ�뺬0.1mol FeBr2����Һ�� |
| �� | ������SO2ͨ�뵽������ˮ | ����SO2ͨ�������İ�ˮ |
| �� | �� | |
| �� | ||
| �� | ||
| �� | ||
| �� |
| A�� | ������ | B�� | ��ë̺ | C�� | ����ơ���� | D�� | ���Ͻ� |
| ��ѧ��Ӧ | ƽ�ⳣ�� | �¶ȡ� | |
| 500 | 800 | ||
| ��2H2��g��+CO��g��?CH3OH��g�� | K1 | 2.5 | 0.15 |
| ��H2��g��+CO2��g��?H2O��g��+CO��g�� | K2 | 1.0 | 2.50 |
| ��3H2��g��+CO2��g��?CH3OH��g��+H2O��g�� | K3 | ||
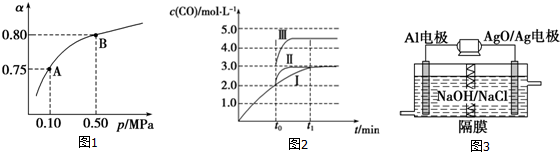
��2��ij�¶��·�Ӧ����H2��ƽ��ת���ʣ���������ϵ��ѹǿ��p���Ĺ�ϵ��ͼ1��ʾ����ƽ��״̬��A�䵽Bʱ��ƽ�ⳣ��KA=KB�����������������=�������ݷ�Ӧ����ڿ��Ƶ���K1��K2��K3֮��Ĺ�ϵ����K3=K1•K2����K1��K2��ʾ������Ӧ��Ϊ���ȷ�Ӧ������ȡ����ȡ�����
��3����3L�ݻ��ɱ���ܱ������з�����Ӧ�ڣ���֪c��CO���뷴Ӧʱ��t�仯���ߢ���ͼ2��ʾ������t0ʱ�̷ֱ�ı�һ�����������ߢ��Ϊ���ߢ�����ߢ�
�����ߢ��Ϊ���ߢ�ʱ���ı�������Ǽ��������
�����ߢ��Ϊ���ߢ�ʱ���ı�������ǽ��������������ѹ����2L��
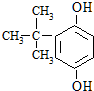
��4���״�ȼ�ϵ�����Ź㷺����;��ͬʱAl-AgO�����Ӧ�ù㷺������أ���ԭ����ͼ3��ʾ����õ�صĸ�����Ӧʽ��Al-3e-+4OH-=AlO2-+2H2O��
��5��һ�������¼״���һ����̼��Ӧ���Ժϳ����ᣮͨ��״���£���a mol•L-1�Ĵ�����b mol•L-1 Ba��OH��2��Һ�������ϣ���Ӧƽ��ʱ��2c��Ba2+��=c��CH3COO-�����ú�a��b�Ĵ���ʽ��ʾ�û����Һ�д���ĵ��볣��Ϊ$\frac{2b}{a-2b}��1{0}^{-7}$L/mol��
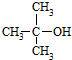 ��
�� ��
��