��Ŀ����
����Ŀ������ij��̼�Ͻ�(����̼���ֵ��ʵĻ����)��ij��ѧ��ȤС��Ϊ�˲ⶨ��̼�Ͻ�������������������̽��Ũ�����ijЩ���ʣ��������ͼ��ʾ��ʵ��װ�ã��г�������ʡ�ԣ���ʵ�鷽������ʵ��̽����
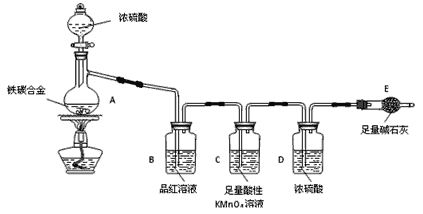
I���ⶨ��������������
��1���������װ�������Ե�һ�ַ����ǣ��رշ�Һ©���Ļ�������Eװ�ú�������һ�����ܣ�Ȼ��___________________________________________________________________����֤��װ�õ����������á�
��2������E������������a g��̼�Ͻ���Ʒ����װ��A�У��ټ���������Ũ���ᣬ��A�в����ݳ�����ʱ��ֹͣ���ȣ�����E�����أ�E����b g����̼�Ͻ���������������Ϊ______________________(д����ʽ)��
��3��װ��C������______________________________________________��s5
��4����ͬѧ��Ϊ�����ݴ�ʵ���õ����ݣ�����Ͻ������������������ܻ�ƫ�ͣ�ԭ���ǿ�����CO2��ˮ��������E��ʹb��������Ϊ�Ľ��ķ�����________________________________________��
��5����ͬѧ��Ϊ����ʹ��ͬѧ��Ϊ��ƫ��õ��Ľ������ݴ�ʵ���úϽ���������������Ҳ���ܻ�ƫ�ߡ�����Ϊ���е�ԭ����________________________________________________________��
��̽��Ũ�����ijЩ���ʣ�
��6����A�еμ�������Ũ���ᣬδ��ȼ�ƾ���ǰ��A��B��������������ԭ���ǣ�_______________________________________��
��7��A������Ũ���ᷢ����Ӧ�Ļ�ѧ����ʽ��_______________________��
���𰸡� �ѵ��ܷ���ʢ��ˮ��ˮ���У�����ƿ��������ܿ������ݲ�����ֹͣ���Ⱥܲ���һ��ˮ�� ![]() ��100% ��ȥCO2�е�SO2 ��װ��E�ĺ��������һ����E��ͬ��װ�� ��Ӧ������CO2����δ����ȫ�ŵ�װ��E�У�����bƫ�� �����£�Fe��ŨH2SO4�ۻ���̼����ŨH2SO4��Ӧ 2Fe �� 6 H2SO4(Ũ)
��100% ��ȥCO2�е�SO2 ��װ��E�ĺ��������һ����E��ͬ��װ�� ��Ӧ������CO2����δ����ȫ�ŵ�װ��E�У�����bƫ�� �����£�Fe��ŨH2SO4�ۻ���̼����ŨH2SO4��Ӧ 2Fe �� 6 H2SO4(Ũ)![]() Fe2(SO4)3 �� 3SO2�� �� 6H2O
Fe2(SO4)3 �� 3SO2�� �� 6H2O
������������ʵ�鷽����������ۣ���1�������װ�õ������ԣ�������ʾ�����õ������Ƽ��������������Եķ��������ѵ��ܷ���ʢ��ˮ��ˮ���У�����ƿ��������ܿ������ݲ�����ֹͣ���Ⱥܲ���һ��ˮ������2������C�ڼ��������£���Ũ���ᷴӦ����SO2��CO2��Ʒ����Һ�����Ǽ���SO2���������Ը��������Һ��ȥSO2��Ũ���������������ʯ���������ղ�����CO2������̼Ԫ���غ㣬n(C)=n(CO2)=b/44mol������������Ϊ(a��b��12/44)g=(11a��3b)/11g��������������Ϊ(11a��3b)/11a����3����������������������ص������dz�ȥCO2�е�SO2����4����װ��E�ĺ��������һ����E��ͬ��װ�� ����5��ƫ�ߵ�ԭ����CO2δ����ȫ�����գ���ɼ����C������ƫ�ͣ�����������ƫ�ߣ����ԭ���ǣ���Ӧ������CO2����δ����ȫ�ŵ�װ��E�У�����bƫ�ͣ���6�������£�Fe��ŨH2SO4�ۻ���̼����ŨH2SO4��Ӧ����7��Ũ��������ǿ�����ԣ���Fe������Fe3�������᱾������ԭ��SO2����Ӧ����ʽΪ��2Fe��6H2SO4(Ũ)![]() Fe2(SO4)3��3SO2����6H2O��
Fe2(SO4)3��3SO2����6H2O��