��Ŀ����
����Ŀ���Ͽ�1 mol AB(g)�����еĻ�ѧ��ʹ��ֱ�������̬Aԭ�Ӻ���̬Bԭ�������յ�������ΪA��B���ļ��ܡ��±��г���һЩ��ѧ���ļ���E��
��ѧ�� | H��H | Cl��Cl | O===O | C��Cl | C��H | O��H | H��Cl |
E/kJ��mol��1 | 436 | 247 | x | 330 | 413 | 463 | 431 |
��ش��������⣺
��1����ͼ��ʾij��Ӧ�������仯��ϵ����˷�ӦΪ (����ȡ����ȡ�)��Ӧ�����Ц�H=
(�ú���a��b�Ĺ�ϵʽ��ʾ)��
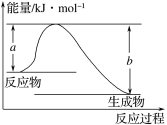
��2����ͼʾ�б�ʾ��ӦH2(g)��![]() O2(g)===H2O(g) ��H=��241.8 kJ��mol��1����b= kJ��mol��1��x= ��
O2(g)===H2O(g) ��H=��241.8 kJ��mol��1����b= kJ��mol��1��x= ��
��3����ʷ�����á��ؿ���������������һ��������CuCl2����������450 �����ÿ����е��������Ȼ��ⷴӦ����������Ӧ�Ļ�ѧ����ʽΪ ���������¶Ⱥ�ѹǿ�Է�Ӧ�ȵ�Ӱ�죬���������е��й����ݣ����㵱��Ӧ����1 mol����ת��ʱ����Ӧ�������仯Ϊ ��
��������1��������1�֣� (a��b)kJ��mol��1��2�֣�
��2��926��3�֣� 496.4��3�֣�
��3��O2��4HCl![]() 2Cl2��2H2O��3�֣� �ų�������2�֣� 31.4 kJ��3�֣�
2Cl2��2H2O��3�֣� �ų�������2�֣� 31.4 kJ��3�֣�
����������1����Ӧ��������������������Ƿ��ȷ�Ӧ����Ӧ��Ϊ��Ӧ��ϼ����յ�������������ɼ��ų�������֮�����H=(a��b)kJ��mol��1��
��2��b��ʾH��Oԭ�ӽ��Ϊ��̬ˮʱ�������仯������ֵΪ463��2=926��436��![]() x��926=��241.8����x=496.4����3������������д����ѧ����ʽ����Ӧ�Ħ�H=(496.4��431��4��247��2��463��4)kJ��mol��1=��125.6 kJ��mol��1����ת��1 mol ����ʱ��Ӧ�ų�������Ϊ31.4 kJ��
x��926=��241.8����x=496.4����3������������д����ѧ����ʽ����Ӧ�Ħ�H=(496.4��431��4��247��2��463��4)kJ��mol��1=��125.6 kJ��mol��1����ת��1 mol ����ʱ��Ӧ�ų�������Ϊ31.4 kJ��

����Ŀ��������һ�����͵���ɫ��Դ������һ����Ҫ�Ļ���ԭ�ϡ���������� (��C �ƣ���ˮ������Ӧ��ȡH2���ֵͺ��ܣ���Ч�ʵ���H2�������÷���������¯����H2��ȼ��¯����CaO�������ɡ�����¯���漰���ķ�ӦΪ��
I C(s)+H2O(g)![]() CO(g)+H2(g) K1��
CO(g)+H2(g) K1��
II CO(g)+H2O(g)![]() CO2(g)+H2(g) K2��
CO2(g)+H2(g) K2��
III CaO(s)+CO2(g)![]() CaCO3(s) K3��
CaCO3(s) K3��
ȼ��¯���漰���ķ�ӦΪ��
IV C(s)+O2(g)=CO2
V CaCO3(s)=CaO(s)+CO2(g)
��1���ù�����H2�ܷ�Ӧ�ɱ�ʾΪC(s)+2H2O(g)+CaO(s) ![]() CaCO3(s)+2H2(g)���䷴Ӧ��ƽ�ⳣ��K=__________����K1��K2��K3�Ĵ���ʽ��ʾ������2L���ܱ������м���һ������C(s)��H2O(g)��CaO(s)��������˵����Ӧ�ﵽƽ�����_______________________��
CaCO3(s)+2H2(g)���䷴Ӧ��ƽ�ⳣ��K=__________����K1��K2��K3�Ĵ���ʽ��ʾ������2L���ܱ������м���һ������C(s)��H2O(g)��CaO(s)��������˵����Ӧ�ﵽƽ�����_______________________��
A.������ѹǿ���ٱ仯 B. H2��H2O(g)�����ʵ���֮�Ȳ��ٱ仯
C.���������ܶȲ��ٱ仯 D.�γ�a molH-H����ͬʱ����2amolH-O��
��2�����ڷ�ӦI����ͬ�¶Ⱥ�ѹǿ��H2����Ӱ�����±���
�¶� | ѹǿp1/Mpa | ѹǿp2/Mpa |
500�� | 45.6% | 51.3% |
700�� | 67.8% | 71.6% |
����ͼ����ȷ����__________________��

��3����֪��Ӧ��Ħ�H=-41.1kJ/mol��C=O��O-H��H-H�ļ��ֱܷ�Ϊ 803 kJ/mol��464 kJ/mol��436 kJ/mol����CO��̼�����ļ���Ϊ___________ kJ/mol��
��4�����ڷ�Ӧ����ƽ��ʱ�ٳ���CO2��ʹ��Ũ������ԭ����2������ƽ���ƶ�����Ϊ___________��������ƽ���CO2Ũ��___________������ ��С���� ���䡱����
��5���״�ȼ�ϵ���Dz��ò����缫�������乤��ԭ����ʾ��ͼ���£�

��ش��������⣺
��Pt(a)�缫�ǵ�ص�_______�����缫��ӦʽΪ______________________��
�ڳ����£��ô˵���Զ��Ե缫���0.5L����ʳ��ˮ��������������������������1.12L��������Ϊ��״���µ���������������Һ��pH=_________��������Һ������仯����