��Ŀ����
6��������ʵ���ҳ��õIJ���������
��ش��������⣺
��1�����Ϊ��͢�������ֱ�Ϊ����ƿ�������ܣ��������ơ���ͬ����
��2��������Ӧ�����ҿ�ֱ�Ӽ��ȵ����Թܣ�
��3���ڷ�Һ�����У������õ������Ǣڢޣ�����š���ͬ����
��4��������һ�����ʵ���Ũ�ȵ���Һʱ����Ҫ���������еĢڢܢ�ᣮ
��5�������ϱ����¶ȵ��Ǣ�ᣮ
��6��ͼ2���¶ȼơ���Ͳ���ζ��ܵ�һ���֣��������������߿̶ȣ���˵����ȷ����b��
a��������Ͳ������Ϊ2.5mL
b��������Ͳ������Ϊ2.5mL
c�����ǵζ��ܣ�����Ϊ2.5mL
d�������¶ȼƣ�������2.5��
��7��ijͬѧ���ô���ʯ��ϡ���ᷴӦ��ȡ����CO2����ʦָ������������װ����ȡ���˷Ѵ�����ϡ���ᣮ���Ǹ�ͬѧѡ���������١����е�һ������������������װ���У������������⣮���㽫��Ҫ���ӵ���������ͼ3�еĺ���λ�ã�
���� ��1����Ϥ����ʵ���������˽����ƣ�
��2���˽ⳣ�����������ú��÷��������˽��ֱ�Ӽ��ȵ������У��Թܡ�������������ȼ�ճף��ձ���Ҫ��ʯ�������ȣ�
��3����Һ©������Ҫ�õ��ձ��ͷ�Һ©����
��4����������һ�����ʵ���Ũ�ȵ���Һ���������ͼʾ��������Һ��Ҫ����ƿ�����������ձ�����Ͳ�ȣ�
��5�����ݶ����������¶�Ҫ�������ȣ���Ҫ�ڳ����½��У�������ƿ���ζ��ܡ���Ͳ�ȣ�
��6��������Ͳ���ζ��ܡ��¶ȼƵĹ��켰ȷ�Ƚ����жϣ�
��7��©����̫�̣������Ķ�����̼������©����й¶����©�����¼�һֻС�Թܣ��Թ��ڼ���ϡ�����©�����⣬������������������ʯ��Ӧ�����н��
��� �⣺��1���ࡢ�����������Ʒֱ�Ϊ����ƿ�������ܣ�
�ʴ�Ϊ������ƿ�������ܣ���
��2����ֱ�Ӽ��ȵ��������Թܣ�
�ʴ�Ϊ���Թܣ�
��3����Һ©������Ҫ�õ��ձ��ͷ�Һ©����
�ʴ�Ϊ���ڢޣ�
��4������һ�����ʵ���Ũ�ȵ���Һ�����У����㡢��������ȡ�����ܽ⡢ת�ơ�ϴ�ӡ����ݡ�ҡ�ȵȣ�ʹ�õ��������У�������ƽ������Ͳ�����ձ�������������ͷ�ιܡ�����ƿ�ȣ�����ʹ�õ�������Ϊ�����ձ����ܽ�ͷ�ιܡ�������ƿ������Ͳ��
�ʴ�Ϊ���ڢܢ�
��5�������������¶�Ҫ�������ȣ���Ҫ�ڳ����½��У�������ƿ����Ͳ�ȣ��������¶ȱ�־���Т�����ƿ������Ͳ��
�ʴ�Ϊ������
��6��a������Ͳû��0�̶ȣ�ͼ����̶������п̶ȣ�Ӧ�����¶ȼƣ���a����
b����û����̶ȣ�����Ͳ��������2.5mL����b��ȷ��
c������̶����Ϸ����ǵζ��ܣ�������2.50mL����c����
d��������Ͳ�������¶ȼƣ���d����
��ѡb��
��7��©����̫�̣������Ķ�����̼������©����й¶����©�����¼�һֻС�Թܣ��Թ��ڼ���ϡ�����©�����⣬������������������ʯ��Ӧ����ȡ������̼����װ���װ��ͼΪ��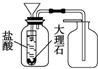 ��
��
�ʴ�Ϊ�� ��
��
���� ���⿼�������������ƺ�װ��ͼ�Ļ��ƣ���Ŀ�ѶȲ�������ʵ���Ҫ���Ŀ���ǽ��Ĺؼ���ע�ⳣ��֪ʶ�Ļ��ۣ�

 Сѧ��ĩ���100��ϵ�д�
Сѧ��ĩ���100��ϵ�д� ��ĩ��ϰ���ϵ�д�
��ĩ��ϰ���ϵ�д� ����ѧ�䵥Ԫ������ĩר����100��ϵ�д�
����ѧ�䵥Ԫ������ĩר����100��ϵ�д� �Ƹ�360�ȶ����ܾ�ϵ�д�
�Ƹ�360�ȶ����ܾ�ϵ�д� ��˵����ȷ���ǣ�������
��˵����ȷ���ǣ�������| A�� | ����ԭ�ӿ��ܶ���ͬһƽ���� | B�� | �����9��̼ԭ����ͬһƽ���� | ||
| C�� | ֻ������5��̼ԭ����ͬһֱ���� | D�� | ��7��̼ԭ�ӿ�����ͬһֱ���� |
| A�� | ���й��ۼ��Ļ�����һ���ǹ��ۻ����� | |
| B�� | ����̬���ʷ����У���һ�������Ź��ۼ� | |
| C�� | ԭ�Ӿ����У�ֻ���ڹ��ۼ� | |
| D�� | �������Ӽ��Ļ�����������ӻ����� |
| A�� | 75%��������������Ҵ���Һ������ҽ������ | |
| B�� | ú����������Һ���������仯����ת��Ϊ���ȼ�� | |
| C�� | �����ղ�����ζ�ķ�������������֯��ʹ���ë֯�� | |
| D�� | ����Cu��OH��2����Һ��ҽԺ�г��������ǵļ�� |
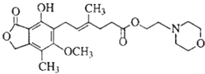
| A�� | MMF����̼������Һ��Ӧ�ų����� | |
| B�� | 1molMMF����6mol����������ȫ�ӳ� | |
| C�� | MMF�����Ȼ�����Һ����ɫ | |
| D�� | 1molMMF����2molNaOH��Һ��ȫ��Ӧ |
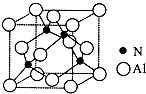 ��Fe3+������SCN-��CN-��F-���л����ӵ��γɺܶ��������ش��������⣺
��Fe3+������SCN-��CN-��F-���л����ӵ��γɺܶ��������ش��������⣺