��Ŀ����
��12�֣� �ش��������⣺
��1����ҵ�ϳɰ���ԭ���ǵ����������������Ǵӿ����з�������ģ�ͨ��ʹ�õ����ַ��뷽���������������������� �ͽ�������̼��Ӧ���ȥCO 2����������Դ����ˮ��̼�⻯���ﷴӦ������CO, д��������Ȼ��Ϊԭ����ȡ�����Ļ�ѧ��Ӧ����ʽ���������������������������� ������������������������������������������������
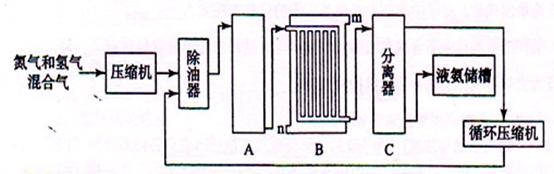
��2���豸A�к��е����������ú���Ƚ��������豸A�з����Ļ�ѧ��Ӧ����ʽΪ��������������������������������������������
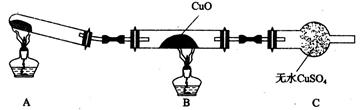
��3�� �豸B�������������������� ������m��n������ͨˮ�ڣ���ˮ������������ ���m����n������
��4���豸C�������������������������������������������� ��
��5����ԭ�����Ʊ������л���CO�Դ����ж������ã�����ȥԭ�����е�CO����ͨ�����·�Ӧ��ʵ�֣�CO(g)+H2O(g) ��CO2 (g)+ H2 (g)����֪1000Kʱ�÷�Ӧ��ƽ�ⳣ��K=0.627����ҪʹCO��ת������90%������ʼ����c(H2O)��c(CO)����������������
��CO2 (g)+ H2 (g)����֪1000Kʱ�÷�Ӧ��ƽ�ⳣ��K=0.627����ҪʹCO��ת������90%������ʼ����c(H2O)��c(CO)����������������
��������������λ��Ч���֣�

��1����ҵ�ϳɰ���ԭ���ǵ����������������Ǵӿ����з�������ģ�ͨ��ʹ�õ����ַ��뷽���������������������� �ͽ�������̼��Ӧ���ȥCO 2����������Դ����ˮ��̼�⻯���ﷴӦ������CO, д��������Ȼ��Ϊԭ����ȡ�����Ļ�ѧ��Ӧ����ʽ���������������������������� ������������������������������������������������
��2���豸A�к��е����������ú���Ƚ��������豸A�з����Ļ�ѧ��Ӧ����ʽΪ��������������������������������������������
��3�� �豸B�������������������� ������m��n������ͨˮ�ڣ���ˮ������������ ���m����n������
��4���豸C�������������������������������������������� ��
��5����ԭ�����Ʊ������л���CO�Դ����ж������ã�����ȥԭ�����е�CO����ͨ�����·�Ӧ��ʵ�֣�CO(g)+H2O(g)
 ��CO2 (g)+ H2 (g)����֪1000Kʱ�÷�Ӧ��ƽ�ⳣ��K=0.627����ҪʹCO��ת������90%������ʼ����c(H2O)��c(CO)����������������
��CO2 (g)+ H2 (g)����֪1000Kʱ�÷�Ӧ��ƽ�ⳣ��K=0.627����ҪʹCO��ת������90%������ʼ����c(H2O)��c(CO)���������������� ��������������λ��Ч���֣�
(1)�ȼ�ѹҺ���ٷ���������2�֣��� CH4��H2O CO��3H2 ��2�֣�
CO��3H2 ��2�֣�
(2) N2(g)��3H2(g) 2NH3 ��2�֣�
2NH3 ��2�֣�
(3)��ȴ��(��������)�� ��1�֣� n����1�֣�
(4)��Һ����δ��Ӧ��ԭ�������루2�֣�
(5)13.8��2�֣�
 CO��3H2 ��2�֣�
CO��3H2 ��2�֣�(2) N2(g)��3H2(g)
 2NH3 ��2�֣�
2NH3 ��2�֣�(3)��ȴ��(��������)�� ��1�֣� n����1�֣�
(4)��Һ����δ��Ӧ��ԭ�������루2�֣�
(5)13.8��2�֣�
�����������1��������������ķ������ȼ�ѹҺ���ٷ���������Ȼ���Ʊ�������������ˮ��Ӧ�������Ǵ�������2��AΪ��Ӧ�ң�װ���ṩ���´����ķ�Ӧ��������Ϊ������������Ӧ�Ʊ������ķ�Ӧ����3��C���Һ�����棬C���轫Һ���������������BΪ��ȴ����Ϊ�Ӵ���ȴЧ�ʣ�װ������˺ܶ���ܵ�����4����Һ�����������ʷ��뿪����5����CO��H2O��ʼŨ��Ϊx��ymol/L,��ת����CO����СֵΪ0.9x��
CO(g)+H2O(g) = CO2(g)+H2(g)
��ʼ x y 0 0
�仯 0.9x 0.9x 0.9x 0.9x
ƽ�� 0.9x y-0.9x 0.9x 0.9x?
����ƽ�ⳣ����ʽ����0.9x��2/0.1x��y-0.9x��=0.627?���?y/x=13.8������ֻҪ��ֵ����13.8,ת���ʾͻᳬ��90%��
�����������Ժϳɰ���ӦΪ����?�ۺϿ����˻�ѧ�뼼��ģ�������֪ʶ��

��ϰ��ϵ�д�
�����Ŀ
 =====
=====