��Ŀ����
(9��)ijУ�о���ѧϰС���ͬѧѧϰ�굪���й����ʵ�����֮�Ե�Ԫ�ص��⻯��NH3���ʵ�̽����
(1)ʵ������ȡ�����Ļ�ѧ����ʽΪ ��
(2)ijͬѧģ���ű���ʳ��ˮ�ռ������ķ����������ű����Ȼ����Һ�ķ����ռ�����������Ϊ���ܷ�ﵽĿ�ģ� (��ܡ���)�������� ��
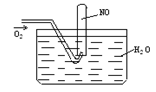
(3)��С���ͬѧ�������ͼ��ʾ��ʵ��װ��(�гּ�β������װ��δ����)��̽�������Ļ�ԭ�ԡ�
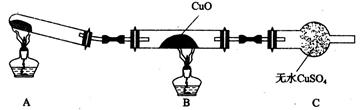
�ٸ�װ�����������һ��ȱ�ݡ�Ϊ��֤ʵ������ȷ�ԣ��Ը�װ�õĸĽ���ʩ��
��
�����øĽ����װ�ý���ʵ�飬CuO��Ϊ��ɫ���ʣ���ˮCuSO4������ͬʱ����һ������Ⱦ�����塣������CuO��Ӧ�Ļ�ѧ����ʽΪ ��
����ͬѧ��ΪNH3��CuO��Ӧ���ɵĺ�ɫ�����п��ܺ�Cu2O��Cu2O��������Һ��Cu+�绯����Cu��Cu2+�������һ����ʵ�����ú�ɫ�������Ƿ���Cu2O
��
(1)ʵ������ȡ�����Ļ�ѧ����ʽΪ ��
(2)ijͬѧģ���ű���ʳ��ˮ�ռ������ķ����������ű����Ȼ����Һ�ķ����ռ�����������Ϊ���ܷ�ﵽĿ�ģ� (��ܡ���)�������� ��
(3)��С���ͬѧ�������ͼ��ʾ��ʵ��װ��(�гּ�β������װ��δ����)��̽�������Ļ�ԭ�ԡ�

�ٸ�װ�����������һ��ȱ�ݡ�Ϊ��֤ʵ������ȷ�ԣ��Ը�װ�õĸĽ���ʩ��
��
�����øĽ����װ�ý���ʵ�飬CuO��Ϊ��ɫ���ʣ���ˮCuSO4������ͬʱ����һ������Ⱦ�����塣������CuO��Ӧ�Ļ�ѧ����ʽΪ ��
����ͬѧ��ΪNH3��CuO��Ӧ���ɵĺ�ɫ�����п��ܺ�Cu2O��Cu2O��������Һ��Cu+�绯����Cu��Cu2+�������һ����ʵ�����ú�ɫ�������Ƿ���Cu2O
��
(1)2NH4Cl+Ca(OH)2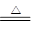 CaCl2+2NH3��+2H2O(2��)
CaCl2+2NH3��+2H2O(2��)
(2)��(1��) ������������ˮ���Ȼ�臨�����ˮ�е��ܽ�Ӱ�첻��(1��)
(3)����װ��A��B֮������װ�м�ʯ�ҵĸ����(1��)
��3CuO+2NH3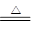 3Cu+N2+3H2O(2��)
3Cu+N2+3H2O(2��)
��ȡ������Ʒ������ϡH2SO4������Һ������ɫ��˵������Cu2O����֮��û��(2��)
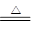 CaCl2+2NH3��+2H2O(2��)
CaCl2+2NH3��+2H2O(2��)(2)��(1��) ������������ˮ���Ȼ�臨�����ˮ�е��ܽ�Ӱ�첻��(1��)
(3)����װ��A��B֮������װ�м�ʯ�ҵĸ����(1��)
��3CuO+2NH3
 3Cu+N2+3H2O(2��)
3Cu+N2+3H2O(2��)��ȡ������Ʒ������ϡH2SO4������Һ������ɫ��˵������Cu2O����֮��û��(2��)
�����������1��ʵ������ȡ�����Ļ�ѧ����ʽΪ2NH4Cl+Ca(OH)2
 CaCl2+2NH3��+2H2O��
CaCl2+2NH3��+2H2O����2�����ڰ�����������ˮ���Ȼ�臨�����ˮ�е��ܽ�Ӱ�첻�����Բ������ű����Ȼ����Һ�ķ����ռ�������
��3�����������ɵİ����к���ˮ�������Ӷ�����ʵ�����֤��������Ҫ��װ��A��B֮������װ�м�ʯ�ҵĸ���������ﰱ����
�ڸ���ԭ���غ��֪��û����Ⱦ������Ӧ���ǵ��������Է�Ӧ�Ļ�ѧ����ʽ��3CuO+2NH3
 3Cu+N2+3H2O��
3Cu+N2+3H2O���۸��������֪��Cu2O��������Һ��Cu+�绯����Cu��Cu2+��ʹ��Һ����ɫ���ݴ˿�����֤������������������ᣬ���Կ����������ϡ���ᡣ�����ȷ�IJ����ǣ�ȡ������Ʒ������ϡH2SO4������Һ������ɫ��˵������Cu2O����֮��û�С�
��������ѧ��һ����ʵ��Ϊ������ѧ�ƣ������л�ѧʵ�鼴��ѧ̽��֮˵������Щ̽���Ժ��Ʊ���ʵ������⣬�ۺ���ǿ�����ۺ�ʵ������ϵ���ܣ��еĻ��ṩһЩ�µ���Ϣ�����Ҫ��ѧ���������桢ϸ�µ����⣬��ϵ��ѧ����֪ʶ�ͼ��ܣ�����֪ʶ����ȡ�Ǩ�ơ����飬ȫ��ϸ�µ�˼�����ܵó���ȷ�Ľ��ۡ�

��ϰ��ϵ�д�
 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
�����Ŀ
 ��CO2 (g)+ H2 (g)����֪1000Kʱ�÷�Ӧ��ƽ�ⳣ��K=0.627����ҪʹCO��ת������90%������ʼ����c(H2O)��c(CO)����������������
��CO2 (g)+ H2 (g)����֪1000Kʱ�÷�Ӧ��ƽ�ⳣ��K=0.627����ҪʹCO��ת������90%������ʼ����c(H2O)��c(CO)����������������