��Ŀ����
����Ŀ������(Na2CO3)�����������о��й㷺����;��������ʵ����ģ���Ƽ�ԭ����ȡNa2CO3������ͼ��
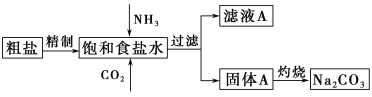
��֪����ʳ��ˮ��ͨ��NH3��CO2�����ķ�ӦΪNaCl��NH3��CO2��H2O=NaHCO3����NH4Cl���ش��������⣺
��1�������к��е�����������Ca2����Mg2����SO42���ȡ����Ƴ��ӵIJ���˳����a��________��________��________��b(����ĸ���)��
a.�����ܽ⣬��ȥ���� b�����������pH c������Ba(OH)2��Һd������Na2CO3��Һ e������
��2����ʳ��ˮ����ͨ��_______����ͨ��________
�Ƶõ�̼������Ʒ��������������NaCl�������ⶨ��Ʒ��Na2CO3������������ij̽����ѧϰС��ֱ����������ʵ�鷽������ش������й����⣺
����һ����һ����������Ʒ�ܽ����������CaCl2��Һ�������ó������ˡ�ϴ�ӡ���ɡ����������㡣
��3��ϴ�ӳ����ľ��������_____________________________��
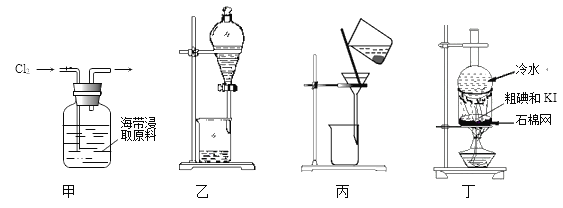
��������������ͼ��ʾװ�����ⶨ������Ʒ��̼���Ƶ���������������̨�����е���ͼ�о�����ȥ����ʵ�鲽�����£�
�ٰ�ͼ����װ�ã�����������ԣ�
��ȷ�Ƶ�ʢ�м�ʯ�ҵĸ����D������Ϊ33.4g��
��ȷ�Ƶ�6g������Ʒ��������b�У�
�ܴ�Һ©��a����������������ϡ���ᣬ�����ٲ�������Ϊֹ��
�ݴ��ɼУ����Թ�A�л���������������ӣ�Ȼ��Ƶø����D��������Ϊ35.6g��
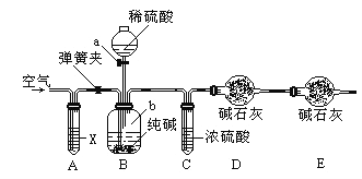
��4��װ��A���Լ�XӦѡ��_________________��
��5������ʵ���в�õ��й����ݣ����㴿����ƷNa2CO3����������Ϊ___________���������С�����һλ����
���𰸡�cdeNH3CO2������ˮ����û�����ʹˮ��Ȼ���£��ظ�2��3��NaOH��Һ88.3%
��������
��1��Ca2����̼���Ƴ�ȥ��Mg2����OH����ȥ��SO42����Ba2����ȥ�������������ữ�������ڹ�����Ba2��Ҫ��̼��������������̼���Ʊ�����������룬������ȷ�IJ���˳����acdeb���ʴ�Ϊ��c��d��e��
��2������NH3������ˮ��CO2��ˮ�е��ܽ�Ȳ���������ͨ�백�������������ܽ�Ȳ����CO2���ʴ�Ϊ��NH3��CO2��
��3��ϴ�ӳ����IJ������ز�������������������м�����ˮ����û�����ʹˮ��Ȼ���£��ظ�����2-3�Σ��ʴ�Ϊ��������ˮ����û�����ʹˮ��Ȼ���£��ظ�2��3�Σ�
��4��װ��A���Լ�X����Ҫ�����dz�ȥ�����еĶ�����̼����ѡ��NaOH��Һ����ȥ���ʴ�Ϊ��NaOH��Һ��
��5����Ӧ�зų�������̼���������m(CO2)=35.6g-33.4g=2.2g��n(CO2)=2.2g��44g/mol=0.05mol�����ݷ���ʽ��Na2CO3+H2SO4=Na2SO4+H2O+ CO2����֪n(Na2CO3)= n(CO2)=0.05mol����m(Na2CO3)= 0.05mol��106g/mol=5.3g�����Դ�����ƷNa2CO3����������=(5.3g��6g����100%��88.3%���ʴ�Ϊ��88.3%��

 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�