��Ŀ����
2012��ʼ������������������Ű���纪�������У�ȼú������β������ɿ�����Ⱦ��ԭ��֮һ��
��1������β����������Ҫԭ��Ϊ��2NO(g) + 2CO(g)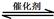 2CO2(g)+ N2(g)����H��0
2CO2(g)+ N2(g)����H��0
�ٸ÷�Ӧƽ�ⳣ������ʽ
�����÷�Ӧ�ھ��ȡ����ݵ��ܱ���ϵ�н��У�����ʾ��ͼ��ȷ����˵����Ӧ�ڽ��е�t1ʱ�̴ﵽƽ��״̬���� ������ţ���
��2��ֱ���ŷ�úȼ�ղ������������������صĻ������⡣
úȼ�ղ����������������������CH4����ԭNOX�������������������Ⱦ��
��֪���� CH4(g)+2NO2(g)��N2(g)��CO2(g)+2H2O(g)����H����867 kJ/mol
�� 2NO2(g)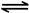 N2O4(g) ��H����56.9 kJ/mol
N2O4(g) ��H����56.9 kJ/mol
�� H2O(g) �� H2O(l) ��H �� ��44.0 kJ��mol
д��CH4����ԭN2O4(g)����N2��H2O(l)���Ȼ�ѧ����ʽ�� ��
��3������ȼ�ϵ�ؿ����������������ʡ���ͼ�����ü���ȼ�ϵ�ص��100mL1mol/Lʳ��ˮ,���һ��ʱ����ռ�����״���µ�����2.24L���������Һ������䣩.
�ټ���ȼ�ϵ�صĸ�����Ӧʽ�� ��
�ڵ�����Һ��pH= (��������������������Һ��Ӧ)
�������������������ڱ�״������ L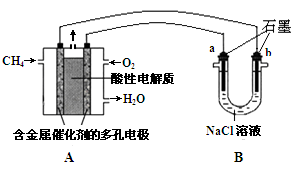
��1����[CO2]2[ N2]/[ NO]2[CO]2�� bd
��2��CH4(g)+N2O4(g) =N2(g) +2H2O(g) + CO2(g) ��H= ��898.1kJ/mol
��3����CH4 ��8e�� + 2H2O =CO2 + 8H+ ��14 �� 1.68L
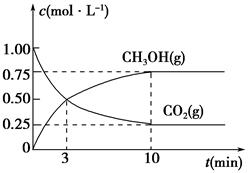
���������������1���ٸ��ݻ�ѧƽ�ⳣ������д���ɣ�������ƽ��Ũ��ϵ�����ݵĻ��ȷ�Ӧ��ƽ��Ũ��ϵ�����ݵĻ���д��[CO2]2[ N2]/[ NO]2[CO]2����a�����淴Ӧ��ƽ�����������������Ҳ��ٱ仯����ͼ��֪��t1ʱ��V������淴Ӧ�������ʷ����仯��δ����ƽ�⣬����b���÷�Ӧ����ӦΪ���ȷ�Ӧ���淴Ӧ�����¶����ߣ���ѧƽ�ⳣ����С������ƽ����¶�Ϊ��ֵ��ƽ�ⳣ�����䣬ͼ����ʵ�ʷ��ϣ���ȷ��c��t1ʱ�̺������̼��NO�����ʵ��������仯��t1ʱ��δ����ƽ��״̬������d�����ŷ�Ӧ�Ľ���NO������������С��t1ʱ��NO�������������䣬����ƽ��״̬����ȷ����bd��
��2����֪���� CH4(g)+2NO2(g)��N2(g)��CO2(g)+2H2O(g)����H����867 kJ/mol �� 2NO2(g)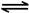 N2O4(g) ��H����56.9 kJ/mol �� H2O(g) �� H2O(l) ��H �� ��44.0 kJ��mol�����ݸ�˹���ɣ���-��+���2��CH4(g)+N2O4(g) =N2(g) +2H2O(g) + CO2(g) ��H= ��898.1kJ/mol��
N2O4(g) ��H����56.9 kJ/mol �� H2O(g) �� H2O(l) ��H �� ��44.0 kJ��mol�����ݸ�˹���ɣ���-��+���2��CH4(g)+N2O4(g) =N2(g) +2H2O(g) + CO2(g) ��H= ��898.1kJ/mol��
��3���ټ���ȼ�ϵ���м����ڸ�������������Ӧ��������������CO2-��H2O�������缫��ӦʽΪCH4 ��8e�� + 2H2O =CO2 + 8H+ ���ڸ�������֪��n(NaCl)=0.1mol�õ�����Ϊ�ȵ�ⱥ��ʳ��ˮ������ˮ�����ݵ�ⷽ��ʽ2Cl- + 2H2O  H2�� + Cl2�� + 2OH-���������ݼ����n(OH-)=0.1mol��Ũ��Ϊ1mol/L��������Һ��pH=14����n(NaCl)=0.1mol�����������������ʵ���Ϊ0.05mol����������õ�����Ϊ�ȵ�ⱥ��ʳ��ˮ������ˮ���缫��ӦʽΪ2H+ + 2e-=H2�� 2Cl- - 2e-=Cl2�� 4OH- - 4e-=O2�� + 2H2O���������������������ʵ���Ϊ0.1mol�����ݵ����غ�ɵã�2n��H2��=2n��Cl-��+4n��O2���������ݼ����n��O2��=0.025mol,��������������0.075mol,���Ϊ1.68L��
H2�� + Cl2�� + 2OH-���������ݼ����n(OH-)=0.1mol��Ũ��Ϊ1mol/L��������Һ��pH=14����n(NaCl)=0.1mol�����������������ʵ���Ϊ0.05mol����������õ�����Ϊ�ȵ�ⱥ��ʳ��ˮ������ˮ���缫��ӦʽΪ2H+ + 2e-=H2�� 2Cl- - 2e-=Cl2�� 4OH- - 4e-=O2�� + 2H2O���������������������ʵ���Ϊ0.1mol�����ݵ����غ�ɵã�2n��H2��=2n��Cl-��+4n��O2���������ݼ����n��O2��=0.025mol,��������������0.075mol,���Ϊ1.68L��
���㣺���黯ѧƽ�ⳣ������ʽ��д����ѧƽ��ͼ��ƽ��״̬�ġ���˹���ɼ��Ȼ�ѧ����ʽ��д��ԭ��ؼ������㡣

�״���һ�ֿ�������Դ�����й㷺�Ŀ�����Ӧ��ǰ����
��1����ҵ��һ������������ַ�Ӧ�ϳɼ״���
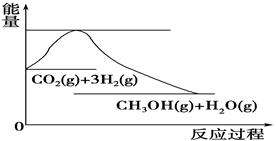
��Ӧ�� CO(g) �� 2H2(g)  CH3OH(g) ��H1
CH3OH(g) ��H1
��Ӧ�� CO2(g) �� 3H2(g)  CH3OH(g) + H2O(g) ��H2
CH3OH(g) + H2O(g) ��H2
���±����������Ƿ�Ӧ���ڲ�ͬ�¶��µĻ�ѧƽ�ⳣ����K����
| �¶� | 250�� | 300�� | 350�� |
| K | 2.041 | 0.270 | 0.012 |
�ɱ��������жϦ�H1 0 �����������������������
��ij�¶��£���2 mol CO��6 mol H2����2L���ܱ������У���ַ�Ӧ���ﵽƽ����c(CO)�� 0.2 mol��L����CO��ת����Ϊ ����ʱ���¶�Ϊ �����ϱ���ѡ��
��2����֪�ڳ��³�ѹ�£�
�� 2CH3OH(l) �� 3O2(g) �� 2CO2(g) �� 4H2O(l) ��H1����1451.6kJ��mol
�� 2CO (g)+ O2(g) �� 2CO2(g) ��H2����566.0kJ��mol
д���״�����ȫȼ������һ����̼��Һ̬ˮ���Ȼ�ѧ����ʽ��
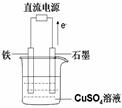
��3��ijʵ��С�����ݼ״�ȼ�յķ�Ӧԭ���������ͼ��ʾ�ĵ��װ�ã�
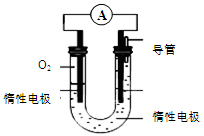
�ٸõ�ص�����ת����ʽΪ ��
�ڸõ�������ĵ缫��ӦΪ ��
�۹���һ��ʱ������Һ��pH��С����õ���ܷ�Ӧ�Ļ�ѧ����ʽΪ ��
��1�� 8gҺ̬��CH3OH����������ȫȼ�գ����ɶ�����̼�����Һ̬ˮʱ�ͷų�Q kJ����������д��Һ̬CH3OHȼ���ȵ��Ȼ�ѧ����ʽ ��
��2���ڻ�ѧ��Ӧ�����У��ƻ��ɻ�ѧ����Ҫ�����������γ��»�ѧ���ֻ��ͷ�������
| ��ѧ�� | H��H | N��H | N��N |
| ����/kJ·mol��1 | 436 | 391 | 945 |
�Ը��ݱ������м������ݼ���a����ֵΪ�� ��
��3����֪��C(s��ʯī)��O2(g)=CO2(g) ��H1����393.5 kJ/mol
2H2(g)��O2(g)=2H2O(l) ��H2����571.6 kJ/mol
2C2H2(g)��5O2(g)=4CO2(g)��2H2O(l) ��H3����2599 kJ/mol
���ݸ�˹���ɣ���C(s��ʯī)��H2(g)����1 mol C2H2(g)��Ӧ���Ȼ�ѧ����ʽ��
��
��֪������Һ��CrO42���Ի�ɫ��Cr2O72-�ԳȺ�ɫ
��PbCrO4������ˮ��Ҳ������ǿ��
��H+(aq��+OH-��aq��=H2O(l���� ��H=" ��a" KJ/mol
3Cl2��g��+2Cr3+��aq��+16OH-(aq��=2CrO42-��aq��+6Cl-��aq��+8H2O(l)����H="��b" KJ/mol
2CrO42-��aq��+2H+(aq�� Cr2O72-��aq��+H2O(l������H="��c" KJ/mol
Cr2O72-��aq��+H2O(l������H="��c" KJ/mol
ƽ�ⳣ��K=9��5��104 ������a��b��c������0��
��������Ӧ�ݣ�ȡ50mL��Һ�������飬���ֲⶨ�������£�
| ʱ�䣨s�� | 0 | 0��01 | 0��02 | 0��03 | 0��04 |
| n ��CrO42������mol�� | 0��01 | 8��0��10-4 | 5��4��10-4 | 5��0��10-4 | |
| n ��Cr2O72������mol�� | 0 | | 4��73��10-3 | | 4��75��10-3 |
�Իش��������⣺
��1��0.02s��0.03s֮����Cr2O72-��ʾ�÷�Ӧ��ƽ����Ӧ����Ϊ
����˵����ȷ�ģ� ��
A��0.03sʱV����CrO42����=2V�棨Cr2O72����
B����ҺpHֵ����˵���÷�Ӧ�Ѵ�ƽ��״̬
C����Һ��c��CrO42������c��Cr2O72����=2:1ʱ�÷�Ӧ�Ѵ�ƽ��״̬
D����Ӧ����2��5��10-3c KJʱCrO42����ת����Ϊ50��
E�����¸÷�Ӧƽ�ⳣ�����
F��0��04sʱ����������Pb��NO3)2 ��ʹ��Һ�ɳ�ɫ��Ϊ��ɫ
��3��0��03sʱ��Һ��pH=
��4����֪����������Cr2O72����Cl-����ΪCl2����������ԭΪCr3+Ϊ���ȷ�Ӧ����д���÷�Ӧ���Ȼ�ѧ����ʽ��
 SO3��g����NO��g��
SO3��g����NO��g��
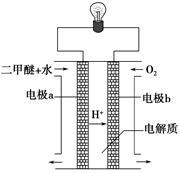
 CH3OH(g)��CO��ƽ��ת�������¶ȡ�ѹǿ�Ĺ�ϵ����ͼ��ʾ����
CH3OH(g)��CO��ƽ��ת�������¶ȡ�ѹǿ�Ĺ�ϵ����ͼ��ʾ����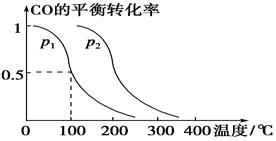
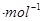 ��
�� ��һ283.0 kJ
��һ283.0 kJ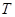 ��
�� ������300�棩��
������300�棩��
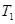 ʱ���ӷ�Ӧ��ʼ����Ӧ�ﵽƽ�⣬���ɼ״���ƽ������Ϊ��
ʱ���ӷ�Ӧ��ʼ����Ӧ�ﵽƽ�⣬���ɼ״���ƽ������Ϊ��
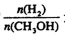 ����
����