��Ŀ����
����Ŀ��������H�Ǻϳ�������Ѫ�ܼ���ҩ����м��壬��ͨ����ͼ��ʾ;���ϳɣ�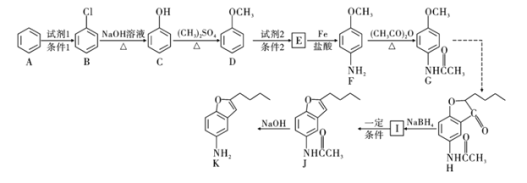
��֪��![]()
![]() ��
��![]() �����ױ�����
�����ױ�����![]() ��
��
![]()
![]() ��
��
�ش��������⣺
��1��д��������D������Ϊ______________��F�еķǺ�������������Ϊ______________
��2��д��![]() �ķ�Ӧ���ͣ�______________��������G�ķ���ʽΪ______________��
�ķ�Ӧ���ͣ�______________��������G�ķ���ʽΪ______________��
��3��д��![]() ��Ӧ�Ļ�ѧ����ʽ��__________________________________________��
��Ӧ�Ļ�ѧ����ʽ��__________________________________________��
��4��������L��Eͬ���칹�壬д��ͬʱ������������L������һ�ֽṹ��ʽ��____________________________��
������![]() ��Һ��Ӧ����ɫ
��Һ��Ӧ����ɫ
�ڱ�����������ȡ����������һ��Ϊ![]()
�۱����ϵ�һ��ȡ��������ͬ���칹��
��5���ϳ�;���У�Fת��ΪG��Ŀ����___________________________________��
��6�����������ϳ�·�ߣ��Ա���![]() Ϊԭ��
Ϊԭ��![]() ���Լ���ѡ
���Լ���ѡ![]() ������Ʊ�
������Ʊ�![]() �ĺϳ�·�ߡ�________________
�ĺϳ�·�ߡ�________________
���𰸡�������![]() ������������������ѡ��������ѡ��ױ���
������������������ѡ��������ѡ��ױ���![]() �Ѽ� ˮ�ⷴӦ
�Ѽ� ˮ�ⷴӦ![]() ��ȡ����Ӧ
��ȡ����Ӧ![]()
![]()
 ��
��
 ��
�� ������������ֹ�ϳɹ����б�����
������������ֹ�ϳɹ����б����� ![]() ��
��
��������
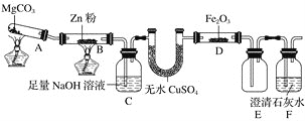
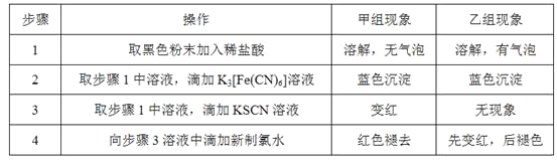
��1��D��![]() ������������
������Ϊ������![]() ������������������ѡ��������ѡ��ױ���
������������������ѡ��������ѡ��ױ���![]() ��FΪ
��FΪ![]() ����Ǻ�������Ϊ������
����Ǻ�������Ϊ������
��2����ͼ ֪��
֪��![]() �ķ�Ӧ����Ϊˮ�ⷴӦ
�ķ�Ӧ����Ϊˮ�ⷴӦ![]() ��ȡ����Ӧ
��ȡ����Ӧ![]() ��GΪ
��GΪ �������ʽΪ
�������ʽΪ![]() ��
��
��3����֪J��������������NaOHˮ��Һ�лᷢ��ˮ�ⷴӦ���䷴Ӧ����ʽΪ�� ��
�� ��
��
��4��L��E��![]() ��ͬ���칹�壬����
��ͬ���칹�壬����![]() ��Һ��Ӧ����ɫ��˵��һ���з��ǻ���������������ȡ����������һ��Ϊ
��Һ��Ӧ����ɫ��˵��һ���з��ǻ���������������ȡ����������һ��Ϊ![]() ��������һ��ȡ��������ͬ���칹�壬�ۺϷ�����������L������
��������һ��ȡ��������ͬ���칹�壬�ۺϷ�����������L������ ��
�� ��
��
��5���ϳ�;���У�Fת��ΪGʱ![]() ������Ӧ����
������Ӧ����![]() ������ת��Ϊ������Ŀ���DZ�����������ֹ���ںϳɹ����б�������
������ת��Ϊ������Ŀ���DZ�����������ֹ���ںϳɹ����б�������
��6������![]() Ϊԭ���Ʊ�
Ϊԭ���Ʊ�![]() �������ɱ�����������Ӧ�������������������������ٷ�����ԭ��Ӧ���ɰ��������������������ȡ����Ӧ��������
�������ɱ�����������Ӧ�������������������������ٷ�����ԭ��Ӧ���ɰ��������������������ȡ����Ӧ��������![]() ����Ӧ������Ϊ��
����Ӧ������Ϊ��![]() ��
��

����Ŀ��Ϊ�˺������û�ѧ�ܣ�ȷ����ȫ���������������Ҫ��ֿ��ǻ�ѧ��Ӧ�ķ�Ӧ�ȣ�����ȡ��Ӧ��ʩ����ѧ��Ӧ�ķ�Ӧ��ͨ����ʵ����вⶨ��Ҳ�ɽ����������㡣
��1��ʵ���ã�5g�״���CH3OH��Һ���������г��ȼ�����ɶ�����̼�����Һ̬ˮʱ�ͷų�113.5kJ�����������ʾ�״���ȼ���ȵ��Ȼ�ѧ����ʽΪ�� ��
��2���������������Ȼ�ѧ����ʽ����a b����������������������������
H2(g)+ 1/2O2(g)��H2O(g) ��H1��a kJ��mol-1
H2(g)+ 1/2O2(g)��H2O(l) ��H2��b kJ��mol-1
��3����1mol��̬������ij�ֹ��ۼ���Ҫ���յ������м��ܡ��ӻ�ѧ���ĽǶȷ�������ѧ��Ӧ�Ĺ��̾��Ƿ�Ӧ��Ļ�ѧ�����ƻ���������Ļ�ѧ�����γɹ��̡��ڻ�ѧ��Ӧ�����У���ѧ����Ҫ�����������γɻ�ѧ���ֻ��ͷ�������
��ѧ�� | H��H | N��H | N��N |
����/kJ��mol��1 | 436 | 391 | 945 |
��֪��ӦN2(g)��3H2(g)![]() 2NH3(g) ��H��a kJ��mol��1���Ը��ݱ������м������ݹ���a��ֵ��_______________(ע����+����������)��
2NH3(g) ��H��a kJ��mol��1���Ը��ݱ������м������ݹ���a��ֵ��_______________(ע����+����������)��
��4�����ݸ�˹���ɿ��Զ�ijЩ����ͨ��ʵ��ֱ�Ӳⶨ�Ļ�ѧ��Ӧ�ķ�Ӧ�Ƚ������㡣����ˮú���ϳɶ����ѵ�������Ӧ���£�
�� 2H2(g) + CO(g)![]() CH3OH(g)����H ����90.8 kJ��mol��1
CH3OH(g)����H ����90.8 kJ��mol��1
�� 2CH3OH(g)![]() CH3OCH3(g) + H2O(g)����H����23.5 kJ��mol��1
CH3OCH3(g) + H2O(g)����H����23.5 kJ��mol��1
�� CO(g) + H2O(g)![]() CO2(g) + H2(g)����H����41.3 kJ��mol��1
CO2(g) + H2(g)����H����41.3 kJ��mol��1
�ܷ�Ӧ��3H2(g) + 3CO(g)![]() CH3OCH3(g) + CO2(g)����H�� ��
CH3OCH3(g) + CO2(g)����H�� ��
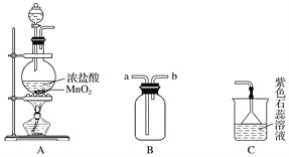
����Ŀ����ͼ����ѧ��ѧ�г����ڻ����ķ�����ᴿ��װ�ã������װ�ûش����⣺
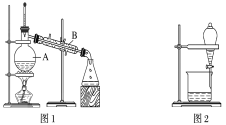
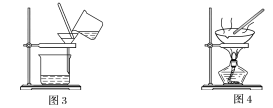
(1)װ��ͼ1��B��������________________��ͼ2��©����������________________��A��һ��Ҫ�������Ƭ����������__________________________�����й�������ʵ�����˵��һ����ȷ����_��
A��ͼ1ʵ���У�����һ��ʱ�����δ�������Ƭ��Ӧ�������ӣ��Է�����Σ��
B��ͼ2ʵ���У�Ӧ���������л��ܼ����¶˵����зų�
C��ͼ3ʵ���У������ò�������©���н��裬�Լӿ�����ٶ�
D��ͼ4ʵ���У����������н϶��������ʱ����ֹͣ����
(2)����һƿA��B�Ļ��Һ����֪���ǵ��������±���
���� | �۵�/�� | �е�/�� | �ܶ�/g��cm��3 | �ܽ��� |
A | ��11.5 | 198 | 1.11 | A��B���ܣ��Ҿ�������ˮ�;ƾ� |
B | 17.9 | 290 | 1.26 |
�ݴ˷�������A��B������ѡ����ͼ�е�ͼ________________��ʾ������
(3)��ͼ2��ʾʵ���У����÷ֲ�������֪����һ��Һ������ˮ�����������һ�ּ����жϷ�����____��