��Ŀ����
1���������ȣ�ClO2������������ˮ�������Դ���Ϊԭ������ClO2�Ĺ�����Ҫ�������ٴ��ξ��ƣ��ڵ������NaCl��Һ����ClO2����ȡ������������ͼ��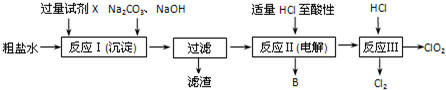
��1����ʳ��ˮ�к���Ca2+��Mg2+��SO42-�����ʣ����Ӳ���ʱ��������ˮ���ȼ���������Լ�X��X��BaCl2���ѧʽ�������������ٲ������ټ��������Na2CO3��NaOH����ַ�Ӧ����һ����ȥ������ⷢ����Һ���Ժ���һ������SO42-����ԭ����BaCl2��BaSO4��BaCO3��Ksp������Һ�д��ڴ�����CO32-����ʱ��BaSO4��s���Ჿ��ת��ΪBaCO3��s��������֪��Ksp��BaSO4��=1.1��10-10��Ksp��BaCO3��=5.1��10-9��
��2�����������У���ʳ��ˮ���ض������µ��õ��������������ᷴӦ����ClO2�����ʱ���ɵ�����B��H2����Ӧ��Ļ�ѧ����ʽΪ2NaClO3+4HCl�T2ClO2��+2NaCl+2H2O��
��3��ClO2�ܲ��ȶ������������ƣ�������ˮ���յõ�ClO2��Һ��Ϊ�ⶨ������Һ��ClO2�ĺ���������������ʵ�飺
����1��ȷ��ȡClO2��Һ10.00mL��ϡ�ͳ�100mL������
����2����ȡV1mL�������뵽��ƿ�У�����������pH��2.0������������KI���壬ҡ�ȣ��ڰ�������30���ӣ�����֪��ClO2+I-+H+-I2+Cl-+H2O δ��ƽ��
����3���Ե�����Һ��ָʾ������c mol•L-1 Na2S2O3��Һ�ζ����յ㣬����Na2S2O3��ҺV2 mL������֪��I2+2S2O32-�T2I-+S4O62-��
��ȷ��ȡ10.00mL ClO2��Һ�IJ�����������ʽ�ζ��ܣ�
�ڵζ������У�������ƽ�вⶨ���ε�ԭ���Ǽ���ʵ����
�۸�����������ɼ����ԭClO2��Һ�����ʵ���Ũ��Ϊ$\frac{2c{V}_{2}}{{V}_{1}}$mol•L-1���ú���ĸ�Ĵ���ʽ��ʾ����
���� ��1����ʳ��ˮ�к���Ca2+��Mg2+��SO42-�����ʣ����Ӳ���ʱ��������ˮ���ȼ���������Լ�BaCl2�����ڳ�ȥSO42-�����������ٲ������ټ��������Na2CO3����ȥԭ��Һ�е�Ca2+��Ϊ��ȥSO42-�������Ba2+��Ȼ���ټ���NaOH��Һ����ȥMg2+����ַ�Ӧ����һ����ȥ������ⷢ����Һ���Ժ���һ������SO42-������Ϊ����Һ�г����ܽ�ƽ�⣬BaSO4 ��BaCO3��Ksp������Һ�д��ڴ�����CO32-����ʱ��BaSO4��s���Ჿ��ת��ΪBaCO3��s����
��2����ʳ��ˮ���ض������µ��õ��������ƣ�����������ԭ��Ӧ�еĻ��ϼ������뽵�͵���������жϵ��ʱ���ɵ�����B�����ƣ��������ⷴӦ��Ϊ���������Ȼ��ⷴӦ���ɶ������ȡ��Ȼ��ƺ�ˮ��
��3����ClO2��Һ�����ԣ�Ӧ������ʽ�ζ���ȷ��ȡ10.00 mL ClO2��Һ��
��Ϊ�˼���ʵ���żȻ�ԣ�����ʵ����ʵ��������ƽ�вⶨ���Σ�ȡ��ƽ��ֵ��
���ɷ���ʽClO2+I-+H+-I2+Cl-+H2O��I2+2S2O32-�T2I-+S4O62-�ù�ϵʽClO2��5S2O32-��n��S2O32-��=cV2��10-3mol������V1mL ClO2����Һ�к��е�ClO2�����ʵ���Ϊ2cV2��10-4mol������c=$\frac{n}{V}$�����ԭClO2��Һ�����ʵ���Ũ�ȣ�
��� �⣺��1�����Ӳ���ʱ��������ˮ���ȼ���������Լ�BaCl2�����ڳ�ȥSO42-�����������ٲ������ټ��������Na2CO3����ȥԭ��Һ�е�Ca2+��Ϊ��ȥSO42-�������Ba2+��Ȼ���ټ���NaOH��Һ����ȥMg2+����ַ�Ӧ����һ����ȥ������ⷢ����Һ���Ժ���һ������SO42-������Ϊ����Һ�г����ܽ�ƽ�⣬BaSO4 ��BaCO3��Ksp������Һ�д��ڴ�����CO32-����ʱ��BaSO4��s���Ჿ��ת��ΪBaCO3��s����
�ʴ�Ϊ��BaCl2��BaSO4��BaCO3��Ksp������Һ�д��ڴ�����CO32-����ʱ��BaSO4��s���Ჿ��ת��ΪBaCO3��s����
��2����ʳ��ˮ���ض������µ��õ��������ƣ�����������ԭ��Ӧ�еĻ��ϼ������뽵�͵�������ȿ�֪���ʱ���ɵ�����BΪH2���������ⷴӦ��Ϊ���������Ȼ��ⷴӦ���ɶ������ȡ��Ȼ��ƺ�ˮ����Ӧ�Ļ�ѧ����ʽΪ��2NaClO3+4HCl�T2ClO2��+2NaCl+2H2O��
�ʴ�Ϊ��H2��2NaClO3+4HCl�T2ClO2��+2NaCl+2H2O��
��3����ClO2��Һ�����ԣ�����ȷ��ȡ10.00 mL ClO2��Һ�IJ�����������ʽ�ζ��ܣ�
�ʴ�Ϊ����ʽ�ζ��ܣ�
���ڼ��Եζ�ʵ�����������������Ϊ�˼���ʵ���żȻ�ԣ�����ʵ����ʵ��������ƽ�вⶨ���Σ�ȡ��ƽ��ֵ���������ӽ�����ֵ��
�ʴ�Ϊ������ʵ����
���ɷ���ʽClO2+I-+H+-I2+Cl-+H2O��I2+2S2O32-�T2I-+S4O62-�ù�ϵʽClO2��5S2O32-��n��S2O32-��=cV2��10-3mol������V1mL ClO2����Һ�к��е�ClO2�����ʵ���Ϊ2cV2��10-4mol����10mL��ԭ��Һ����ClO2�����ʵ���Ϊ��2cV2��10-4mol��$\frac{100mL}{{V}_{1}}$=$\frac{2c{V}_{2}}{{V}_{1}}$��10-2mol������ԭClO2��Һ�����ʵ���Ũ��Ϊ��$\frac{\frac{2c{V}_{2}}{{V}_{1}}��1{0}^{-2}mol}{0.01L}$=$\frac{2c{V}_{2}}{{V}_{1}}$mol/L��
�ʴ�Ϊ��$\frac{2c{V}_{2}}{{V}_{1}}$��
���� ���⿼��������ʵ�鷽������ơ���ѧʵ������������������ۺ�Ӧ�ã���Ŀ�Ѷ��еȣ���ȷʵ��ԭ������ѧʵ�������������Ϊ���ؼ��������ۺ��Խ�ǿ����ֿ�����ѧ���ķ������������������Ӧ�û���֪ʶ��������

 ����ѧ����ϵ�д�
����ѧ����ϵ�д�| A�� | C3N4������ԭ�Ӿ��� | |
| B�� | C3N4�����У�C-N���ļ����Ƚ��ʯ�е�C-C���ļ���Ҫ�� | |
| C�� | C3N4������ÿ��Cԭ������4��Nԭ�ӣ���ÿ��Nԭ������3��Cԭ�� | |
| D�� | C3N4����������ͨ�����Ӽ���� |
| A�� | ��������ΪFe3O4��SiO2 | |
| B�� | �������뻹ԭ�����ʵ�����֮��Ϊ2��3 | |
| C�� | ����1.5mol Fe2SiO4�μӷ�Ӧʱ��ת�Ƶĵ���Ϊ3 mol | |
| D�� | ����1mol CO2�μӷ�Ӧʱ����������Fe2SiO4�����ʵ���Ϊ1mol |
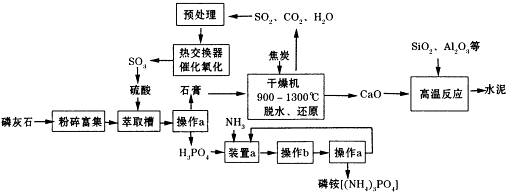
��1��D����a�����ƹ��ˣ�ʵ�����н��д˲���ʱ�õ��IJ���������©�������������ձ�
��2����ʵ�����в���b���������ᾧ����ȴ�ᾧ��
��3��������з�����Ӧ�Ļ�ѧ����ʽΪ2CaSO4•2H2O+C$\frac{\underline{\;����\;}}{\;}$ 2CaO+2SO2��+CO2��+4H2O��
��4��ˮ�ೣ�����������ϣ���������ˮ���ˮӲ�����ʣ�
��5��SO2�Ĵ�������ӦΪ2SO2��g��ʮ02��g��?2S03��g����ʵ����ѹǿ���¶ȶ�S02ת���ʵ�Ӱ�����±���ԭ�������ɷֵ��������ΪSO2��7%��02��11%��N2��82%����
| ѹǿ/Mpa ת����/% �¶�/�� | 0.1 | 0.5 | 1 | 10 |
| 400 | 99.2 | 99.6 | 99.7 | 99.9 |
| 500 | 93.5 | 96.9 | 97.8 | 99.3 |
| 600 | 73.7 | 85.8 | 89.5 | 96.4 |
�ڴ�����ʱʹ���Ƚ�������ԭ������ȥ��Ӧ�зų���������ʹ��Ӧ������彵�²�Ԥ��δ��Ӧ�����壮
��6����������������β�����˺���N2��02�⣬������SO2������S03�����������������ڲⶨ����β����SO2�������Լ�����CD������д��Ӧ��ĸ��
a��NaOH��Һ����̪��Һ b��Na2CO3��Һ����̪��Һ c����ˮ��������Һ d��KMn04��Һ��ϡ���ᣮ
��֪����[Cu��NH3��4]SO4�ڳ������ȶ�������ˮ�л�ֽ�����NH3��
�ڲ��ֽ��������������������������pH��Χ���±���ʾ����ʼ������pH����������Ũ��Ϊ1.0mol•L-1���㣩��
| ��ʼ������pH | ������ȫ��pH | |
| Fe3+ | 1.1 | 3.2 |
| Mg2+ | 8.3 | 9.8 |
| Cu2+ | 4.4 | 6.4 |
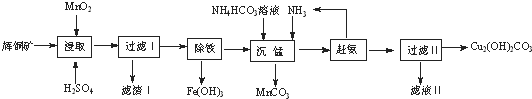
��1���ܼӿ��ȡ���ʵĴ�ʩ�з����ʯ�������¶ȣ����ʵ��������Ũ�Ȼ���裩������д2����
��2����ȡ��õ��Ľ���Һ�к���CuSO4��MnSO4��д����ȡʱ����CuSO4��MnSO4��Ӧ�Ļ�ѧ����ʽ
2MnO2+Cu2S+4H2SO4=S��+2CuSO4+2MnSO4+4H2O����������ijɷ�ΪMnSO4��SiO2��S��
��3�����������ķ�����ͨ��������ҺpH��ʹFe3+ˮ��ת��ΪFe��OH��3��������Լ�A�����ǰ�ˮ���ѧʽ����������ҺpH�ķ�ΧΪ3.2��PH��4.4��
��4�������̡�����Mn2+�������з�����Ӧ�����ӷ���ʽΪMn2++CO32-=MnCO3�������ϰ���ʱ�������˵IJ�������Ϊ���ȣ�
��5���ⶨ��ʽ̼��ͭ���ȿ��õζ�����ȡ6.2500g��Ʒ��100mLС�ձ��У�����20mL����ˮ���裬�ټ���8mL6mol•L-1����ʹ����ȫ�ܽ⣬��ȴ����ת����250mL����ƿ�У���ˮ���ݣ�ҡ�ȣ���ȡ25.00mL��õ���Һ����ƿ�У�����40.00mL0.2000mol•L-1EDTA��Һ��Ȼ�����MnO2������0.2000mol•L��Zn2+����Һ�ζ����յ㣬���ı���Һ18.00mL����֪EDTA��Cu2+��Zn2+�������ʵ�����1��1��Ӧ������Ʒ��Cu2��OH��2CO2����������Ϊ78.14%��
 ij��ѧʵ��С����Ҫ�˽��г�������ʳ�ð״ף���Ҫ�Ǵ����ˮ��Һ����Ũ�ȣ��ִ��г�������һƿijƷ��ʳ�ð״ף���ʵ���ұ�NaOH��Һ������еζ����±���4�ֳ���ָʾ���ı�ɫ��Χ��
ij��ѧʵ��С����Ҫ�˽��г�������ʳ�ð״ף���Ҫ�Ǵ����ˮ��Һ����Ũ�ȣ��ִ��г�������һƿijƷ��ʳ�ð״ף���ʵ���ұ�NaOH��Һ������еζ����±���4�ֳ���ָʾ���ı�ɫ��Χ��| ָʾ�� | ʯ�� | ���� | ���� | ��̪ |
| ��ɫ��Χ��pH�� | 5.0��8.0 | 3.1��4.4 | 4.4��6.2 | 8.2��10.0 |
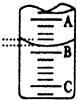
��2����ͼ��ʾ50mL���ζ�����Һ���λ�ã���A��C�̶ȼ����1mL��A���Ŀ̶�Ϊ25���ζ�����Һ�����ӦΪ25.40mL��
��3���ڵζ��Ĺ����У�ʱ�������ἷѹ��ʽ�ζ��ܵIJ��������ֱߵα�����ƿ���۾�Ӧ�۲���ƿ����Һ��ɫ�仯������ƿ����ɫ��Ϊdz��ɫ�Ұ�����ڲ���ԭ���ζ��յ��жϣ���ֹͣ�ζ�����¼�ζ��ܵļ�����
��4��Ϊ�˼�Сʵ������ͬѧһ������������ʵ�飬����ÿ����ȡ�״������ΪVmL��NaOH��ҺŨ��Ϊc mol•L-1������ʵ������¼���£�
| ʵ����� | ��һ�� | �ڶ��� | ������ |
| ����NaOH��Һ���/mL | 26.02 | 25.32 | 25.28 |
A��ʵ�����ʱ���ӿ̶��߶�ȡ�ζ��յ�ʱNaOH��Һ�����
B���ζ�ǰ�ζ��ܼ��������ݣ��ζ�����������
C��ʢװ��Һ�ĵζ���װҺǰ������ˮ��ϴ����δ�ñ�Һ��ϴ
D����һ�εζ��õ���ƿ�ô�װҺ��ϴ����������δ��ϴ
E���μ�NaOH��Һ���죬δ������տ�����Һ��ɫ������ֹͣ�ζ�
��5�������������ݣ�д������ð״��д������ʵ���Ũ�ȵı���ʽ���ػ���$\frac{25.30��c}{V}$��
| A�� | 0.1 mol•L-1��NaHSO3��Һ��0.2 mol•L-1��NaClO��Һ�������ϣ�HSO3-+ClO-=SO42-+Cl-+H+ | |
| B�� | ��Ũ�ȵ�Fe2��SO4��3��Һ��Ba��OH��2��Һ��ϣ�2Fe3++3SO42-+3Ba2++6OH-=2Fe��OH��3��+3BaSO4�� | |
| C�� | Ca��HCO3��2��Һ������NaOH��Һ��Ӧ��2HCO3-+Ca2++2OH-=CaCO3��+CO32-+2H2O | |
| D�� | H218O��Ͷ��������ƣ�2H218O+2Na2O2=4Na++4OH-+18O2�� |