��Ŀ����
18����֪��A��B��F�Ǽ�ͥ�г������л��F������ʳƷ��װ��E��ʯ�ͻ�����չˮƽ�ı�־��������ͼת����ϵ�ش����⣮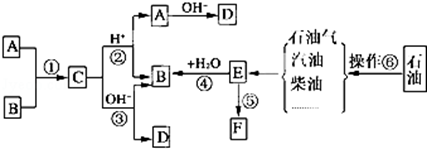
��1���ֱ�д��A��E�й����ŵ����ƣ�A���Ȼ���E��̼̼˫����
��2������������Ϊ���߷��ӻ�����F�ķ��ӽṹ��ʽΪ
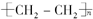 ��
����3��ÿ���ķ�Ӧ���ͷֱ�Ϊ��ȡ����Ӧ�ܼӳɷ�Ӧ�ݼӾ۷�Ӧ��
��4����д�����з�Ӧ�Ļ�ѧ����ʽ��
��д����Ӧ����Ũ������Һ�м��ȷ�Ӧ�ķ���CH3CH2OH+CH3COOH$��_{��}^{Ũ����}$CH3COOCH2CH3+H2O��
��B�ڽ���ͭ�������ڿ����м��ȷ�Ӧ2CH3CH2OH+O2 $��_{��}^{Cu}$2CH3CHO+2H2O��
��5����֪�л���M�Ľṹ��ʽΪ
 ��д��M�ڴ�������������Ϊ�߷��ӻ�����ķ�Ӧ����ʽ
��д��M�ڴ�������������Ϊ�߷��ӻ�����ķ�Ӧ����ʽ $\stackrel{һ������}{��}$
$\stackrel{һ������}{��}$ +��n-1��H2O�������л���N��Ũ��������£����������������һ�ֻ�״��
+��n-1��H2O�������л���N��Ũ��������£����������������һ�ֻ�״�� �����л���N�Ľṹ��ʽΪ��
�����л���N�Ľṹ��ʽΪ�� ��M��Һ���ܺ�Na��Ӧ�����ܺ�NaOH��Һ��Ӧ��д��M��Na��Ӧ�ķ�Ӧ����ʽ��
��M��Һ���ܺ�Na��Ӧ�����ܺ�NaOH��Һ��Ӧ��д��M��Na��Ӧ�ķ�Ӧ����ʽ�� +2Na��
+2Na��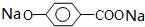 +H2����
+H2����
���� E��ʯ�ͻ�����չˮƽ�ı�־����EΪCH2=CH2��A��B��F�Ǽ�ͥ�г������л��Eͨ���Ӿ۷�Ӧ�õ������ĸ߷��Ӳ���F����FΪ����ϩ��E��ˮ�����ӳɷ�Ӧ����BΪC2H5OH��A��B��Ӧ����C��C��������������ˮ������A��B��D����AΪCH3COOH��CΪCH3COOCH2CH3��DΪCH3COONa���ɲ�����֪��Ϊʯ�ͷ���
��5��M�������۷�Ӧ���� ��N����������Ӧ�õ���������֪NΪ
��N����������Ӧ�õ���������֪NΪ ��M���Ȼ������ǻ�����Na��Ӧ����Ӧ����
��M���Ȼ������ǻ�����Na��Ӧ����Ӧ���� ��������
��������
��� �⣺E��ʯ�ͻ�����չˮƽ�ı�־����EΪCH2=CH2��A��B��F�Ǽ�ͥ�г������л��Eͨ���Ӿ۷�Ӧ�õ������ĸ߷��Ӳ���F����FΪ����ϩ��E��ˮ�����ӳɷ�Ӧ����BΪC2H5OH��A��B��Ӧ����C��C��������������ˮ������A��B��D����AΪCH3COOH��CΪCH3COOCH2CH3��DΪCH3COONa���ɲ�����֪��Ϊʯ�ͷ���
��1��AΪCH3COOH�����й�����Ϊ�Ȼ���EΪ��ϩ�����й�����Ϊ̼̼˫�����ʴ�Ϊ���Ȼ���̼̼˫����
��2����ʯ���и���ֵķе㲻ͬ�������÷���ķ������õ�����֣����Բ�����Ϊ����F�Ľṹ��ʽΪ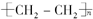 ��
��
�ʴ�Ϊ������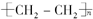 ��
��
��3��������������֪����Ӧ�����Ҵ������ᷢ��������Ӧ��������������Ҳ����ȡ����Ӧ����Ӧ������ϩ��ˮ�����ӳɷ�Ӧ�����Ҵ�����Ӧ������ϩ�����Ӿ۷�Ӧ���ɾ���ϩ��
�ʴ�Ϊ��ȡ����Ӧ���ӳɷ�Ӧ���Ӿ۷�Ӧ��
��4�����Ҵ���������Ũ���ᡢ���������·�Ӧ����������������Ӧ����ʽΪ��CH3CH2OH+CH3COOH$��_{��}^{Ũ����}$CH3COOCH2CH3+H2O��
�ʴ�Ϊ��CH3CH2OH+CH3COOH$��_{��}^{Ũ����}$CH3COOCH2CH3+H2O��
���Ҵ��ڽ���ͭ�������ڿ����м��ȷ�Ӧ����ʽΪ��2CH3CH2OH+O2 $��_{��}^{Cu}$2CH3CHO+2H2O��
�ʴ�Ϊ��2CH3CH2OH+O2 $��_{��}^{Cu}$2CH3CHO+2H2O��
��5�� �������۷�Ӧ����
�������۷�Ӧ���� ����Ӧ����ʽΪ��n
����Ӧ����ʽΪ��n $\stackrel{һ������}{��}$
$\stackrel{һ������}{��}$ +��n-1��H2O��
+��n-1��H2O��
N����������Ӧ�õ���������֪NΪ ��
��
M���Ȼ������ǻ�����Na��Ӧ����Ӧ���� ����������Ӧ����ʽΪ��
����������Ӧ����ʽΪ�� +2Na��
+2Na�� +H2����
+H2����
�ʴ�Ϊ�� $\stackrel{һ������}{��}$
$\stackrel{һ������}{��}$ +��n-1��H2O��
+��n-1��H2O�� ��
�� +2Na��
+2Na�� +H2����
+H2����
���� ���⿼���л�����ƶϡ��л���ṹ�����ʣ�����ƴ������Ŀ���漰ϩ�����������ᡢ����������ת����EΪ��ϩ�ǽ�����ͻ�ƿڣ��ٸ��ݸ�����֮���ת���Ƴ������ʣ��ѶȲ���

| A�� | 2p | B�� | 3f | C�� | 4p | D�� | 5s |
| A�� | ���³�ѹ�£�71 g Cl2����2 NA��Clԭ�� | |
| B�� | ��40 g NaOH��������1 Lˮ�У�������Һ��NaOH�����ʵ���Ũ��Ϊ1 mol•L-1 | |
| C�� | ���ʵ���Ũ��Ϊ1 mol/L��K2SO4��Һ�У���2 NA��K+ | |
| D�� | ���³�ѹ�£�11.2 L N2�к��еķ�����Ϊ0.5 NA |
| A�� | ������H2O��CO��H2�����ʵ���֮��Ϊ1��1��1 | |
| B�� | v����CO��=v����H2O�� | |
| C�� | ����n molCO��ͬʱ����n mol H2 | |
| D�� | 1mol H-H������ͬʱ����2mol H-O�� |
| A�� | BaCl2 NaOH NaHCO3 | B�� | Na2CO3 MgCl2 H2SO4 | ||
| C�� | AlCl3 NH3•H2O NaOH | D�� | Ba��OH��2 CaCl2 Na2SO4 |

| A�� | װ�âٿ����ڷ��뱽���屽�Ļ���� | |
| B�� | װ�âڿ��������հ��������ܹ���ֹ���� | |
| C�� | ��NH4ClΪԭ�ϣ�װ�âۿ������Ʊ�NH3 | |
| D�� | ʣ��װ�âܿ��ռ�Cl2��H2�����壬ʢ��ˮʱ�����ռ�NO������ |
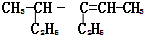 ��ϵͳ������4-��-3-�һ�-2-��ϩ
��ϵͳ������4-��-3-�һ�-2-��ϩ �ķ���ʽ��C4H8O
�ķ���ʽ��C4H8O