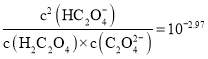��Ŀ����
����Ŀ����1200 ��ʱ����Ȼ���������лᷢ�����з�Ӧ��H2S(g)��![]() O2(g)= SO2(g)��H2O(g)����H1��H2S(g)��
O2(g)= SO2(g)��H2O(g)����H1��H2S(g)��![]() O2(g)= S(g)��H2O(g)����H2��2H2S(g)��SO2(g)=
O2(g)= S(g)��H2O(g)����H2��2H2S(g)��SO2(g)= ![]() S2(g)��2H2O(g)����H3��2S(g)= S2(g)����H4 ����H4����ȷ����ʽΪ�� ��
S2(g)��2H2O(g)����H3��2S(g)= S2(g)����H4 ����H4����ȷ����ʽΪ�� ��
A.��H4��![]() (��H1����H3��3��H2)B.��H4��
(��H1����H3��3��H2)B.��H4��![]() (3��H2����H1����H3)
(3��H2����H1����H3)
C.��H4��![]() (��H1����H3��3��H2)D.��H4��
(��H1����H3��3��H2)D.��H4��![]() (��H1����H3��3��H2)
(��H1����H3��3��H2)
���𰸡�C
��������
Ӧ�ø�˹���ɷ������㡣
�� H2S(g)��![]() O2(g)= SO2(g)��H2O(g)����H1
O2(g)= SO2(g)��H2O(g)����H1
�� H2S(g)��![]() O2(g)= S(g)��H2O(g)����H2
O2(g)= S(g)��H2O(g)����H2
��2H2S(g)��SO2(g)= ![]() S2(g)��2H2O(g)����H3
S2(g)��2H2O(g)����H3
��2S(g)= S2(g)����H4
���ݸ�˹����֪����-3����+�ۣ���![]() �ɵ÷�Ӧ�ܣ���H4=
�ɵ÷�Ӧ�ܣ���H4=![]() (��H1-3��H2+��H3)��
(��H1-3��H2+��H3)��
��ѡC��

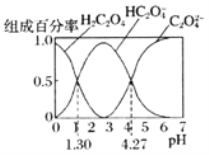
 ��ĩ���100�ִ��½����ȫ�Ծ�ϵ�д�
��ĩ���100�ִ��½����ȫ�Ծ�ϵ�д�����Ŀ������˵����ȷ����![]()
A.![]() ���
�л���![]() �ķ��ӽṹ�к��е�
�ķ��ӽṹ�к��е�![]() ����Ŀһ��Ϊ
����Ŀһ��Ϊ![]()
B.һ���¶��£��Ȼ��ˮ�����ӷ���ʽ��![]() ������
������![]() ��ʾ�����ӻ���
��ʾ�����ӻ���![]() ��ʾ��ˮ���볣�������Ȼ��ˮ��ƽ�ⳣ��
��ʾ��ˮ���볣�������Ȼ��ˮ��ƽ�ⳣ��![]()
C.��֪��Ӧ��![]() ��
��![]() ��
��![]() ������������Һ�������ԣ�
������������Һ�������ԣ�![]()
D.��֪
���ۼ� |
|
|
|
|
���� | 360 | 436 | 431 | 176 |
��Ӧ![]() ���ʱ�Ϊ��
���ʱ�Ϊ��![]()
����Ŀ���״�����Ҫ�Ļ�ѧ��ҵ����ԭ�Ϻ����Һ��ȼ�ϡ���֪�Ʊ��״����йػ�ѧ��Ӧ�Լ��ڲ�ͬ�¶��µĻ�ѧ��Ӧƽ�ⳣ�����±���ʾ��
��ѧ��Ӧ | ƽ�ⳣ�� | �¶�/�� | |
500 | 800 | ||
��2H2(g)��CO(g) | K1 | 2.5 | 0.15 |
��H2(g)��CO2(g) | K2 | 1.0 | 2.50 |
��3H2(g)��CO2(g) | K3 | ||
��1���ݷ�Ӧ����ڿ��Ƶ���K1��K2��K3֮��Ĺ�ϵ����K3��______(��K1��K2��ʾ)��
��2����Ӧ�۵Ħ�H____0(���������)��
��3��500��ʱ��÷�Ӧ����ijʱ��H2(g)��CO2(g)��CH3OH(g)��H2O(g)��Ũ����ȣ��Ҿ�Ϊ0.1mol��L��1�����ʱ����____����(�>������������<��)
��4��ij�¶�����2L�����ܱ������м���CH3OH������Ӧ2CH3OH��g��![]() CH3OCH3��g��+H2O��g��������й��������£�
CH3OCH3��g��+H2O��g��������й��������£�
��Ӧʱ��/min | 0 | 1 | 2 | 3 | 4 |
n��CH3OH��/mol | 1.02 | 0.42 | 0.22 | 0.02 | 0.02 |
�ٷ�Ӧ��2min����CH3OCH3��ʾ�Ļ�ѧ��Ӧ����Ϊ____��
�ڸ��¶��µķ�Ӧ��ƽ�ⳣ��Ϊ____��