��Ŀ����
����Ŀ����ˮCoCl2Ϊ����ɫ����ˮ���Ϊ�ۺ�ɫ��ˮ������ˮ�������Ⱥ��ֱ����ˮCoCl2���ʳ���ʵ������������ʪ���Ϳ���ʪ��ָʾ����
CoCl2��xH2O![]() CoCl2��xH2O
CoCl2��xH2O
����ɫ�������������ۺ�ɫ
����65 g��ˮCoCl2����ˮ����CoCl2��xH2O 119 g��
��1��ˮ������x��________��
��2�����û�������Co2����λ��Ϊ6�����Ҿ������ⶨ��֪�ڽ�����ռ��Cl���ĸ�����Ϊ1��1�����仯ѧʽ�ɱ�ʾΪ________��
���𰸡� 6 [Co(H2O)5Cl]Cl��H2O
�����������صľ���ˮ������Ϊ54g,���ʵ���Ϊ3mol,65g��ˮCoCl2Ϊ0.5mol
CoCl2 -----------------xH2O
0.5mol 3mol
��������Co2+����λ��Ϊ6�����Ҿ��ⶨ��֪�ڽ�����Cl-�ĸ�����Ϊ1��1��
�����������Ϊһ�����ڽ���һ������λ��Ϊ6��ˮ����Ϊ5����Ϊ[CoCl(H2O )5] Cl��H2O

����Ŀ��Y��Z��W��R��M����Ԫ�أ�λ��Ԫ�����ڱ���ǰ�����ڣ����ǵĺ˵��������������������Ϣ��
Ԫ�� | �����Ϣ |
Y | ԭ�Ӻ�����6����ͬ�˶�״̬�ĵ��� |
Z | �ǽ���Ԫ�أ���̬ԭ�ӵ�s����ĵ���������p����ĵ���������ͬ |
W | ����Ԫ�أ���Zԭ�ӵļ۵�������ͬ |
R | �۲�����Ų�ʽΪ3d64s2 |
M | IB�壬�䱻������������ҵ�������� |
��ش��������⣨Y��Z��W��R��M������Ӧ��Ԫ�ط��ű�ʾ��:
��1��Z��WԪ����ȣ���һ�����ܽϴ����_____________________��M2+�ĺ�������Ų�ʽΪ________________________��
��2��M2Z���۵��M2W��_________������������������) �������ԭ��___________ ��
��3��N3-��YZ2�ǵȵ����壬��N3-�ĽṹʽΪ_________________ ��
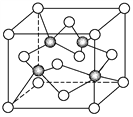
��4��WZ2������Wԭ�Ӽ۲���Ӷ�����_____________�ԣ�WZ2��VSEPR ģ������Ϊ______________________��WZ3��̬Ϊ�����ӣ��÷�����Wԭ�ӵ��ӻ��������Ϊ__________________��WZ3�������廷״�ṹ��ͼ1��ʾ���ýṹ��Wԭ�ӵ��ӻ��������Ϊ__________���ýṹ��W-Z���������࣬һ�����Լ140pm����һ�����ԼΪ160pm���϶̵ļ�Ϊ___________(��ͼ2����ĸ) ���÷����к���___��������
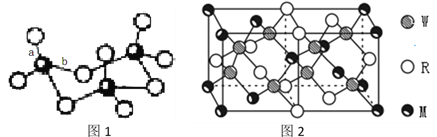
��5��MRW2�ľ�����ͼ2��ʾ����������a=0.524nm��c=1.032nm��MRW2�ľ�����ÿ��Mԭ����_______��Wԭ�������������ܶ���=_______g/cm3��ֻҪ������ʽ�����ؼ������ֵ�������ӵ�����ΪNA��6��02��1023mol-1����
����Ŀ�����ֶ�����Ԫ��A��B��C��D�����ʻ�ṹ��Ϣ���¡�
��Ϣ��:ԭ�Ӱ뾶��С:A>B>C>D
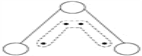
��Ϣ��:����Ԫ��֮���γɵ�ij���ַ��ӵı���ģ�ͼ���������:
|
|
|
�ǵ��������������֮һ,����ΪҺ̬,�ǰ��������������������������Ҫ��Դ,Ҳ������������Ҫ����ɲ��� | ��ɫ,��ζ����ȼ,��21���͵���Ҫ��Դ | ����,��ǿ������,������������ɱ�� |
�����������Ϣ�ش��������⡣
��1���ס��ҡ����к��еĹ�ͬԪ���� (������)��
��2��BԪ�������ڱ��е�λ��Ϊ ��
��3������Ԫ�ص�ԭ��M����һ��δ�ɶ�p���ӵ��� (��Ԫ�ط���)��
��4�����ĵ���ʽΪ ,����SO2ˮ��Һ�ɷ���������ԭ��Ӧ,��������ǿ��,��ѧ��Ӧ����ʽΪ ��
���𰸡���1���⣨2����2������A�壨3��Cl
��4��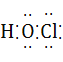 HClO+H2O+SO2��H2SO4+HCl
HClO+H2O+SO2��H2SO4+HCl
��������������������ֶ�����Ԫ��A��B��C��D������Ԫ��֮���γɼס��ҡ������ַ��ӣ�����Ϣ��������ΪV�ͽṹ���ǵ��������������֮һ���ǰ��������������������������Ҫ��Դ��Ҳ������������Ҫ����ɲ��֣��ʼ�Ϊˮ����Ϊ��������ṹ����ɫ��ζ����ȼ����21���͵���Ҫ��Դ����Ϊ���飬��������3����ͬ��ԭ�ӣ����ٻ���C��H��O�е����֣�����ǿ�����ԣ�������������ɱ������Ӧ��HClO���ٸ�����Ϣ��ԭ�Ӱ뾶��С��A��B��C��D�ɵã�AΪClԪ�ء�BΪCԪ�ء�CΪOԪ�ء�DΪHԪ�أ��ݴ˴��⣮
�⣺���ֶ�����Ԫ��A��B��C��D������Ԫ��֮���γɼס��ҡ������ַ��ӣ�����Ϣ��������ΪV�ͽṹ���ǵ��������������֮һ���ǰ��������������������������Ҫ��Դ��Ҳ������������Ҫ����ɲ��֣��ʼ�Ϊˮ����Ϊ��������ṹ����ɫ��ζ����ȼ����21���͵���Ҫ��Դ����Ϊ���飬��������3����ͬ��ԭ�ӣ����ٻ���C��H��O�е����֣�����ǿ�����ԣ�������������ɱ������Ӧ��HClO���ٸ�����Ϣ��ԭ�Ӱ뾶��С��A��B��C��D�ɵã�AΪClԪ�ء�BΪCԪ�ء�CΪOԪ�ء�DΪHԪ�أ�
��1����������ķ�����֪���ס��ҡ����к��й�ͬԪ������Ԫ�أ�
�ʴ�Ϊ���⣻
��2��BΪ̼Ԫ�أ������ڱ��еڶ����ڵ�IVA�壬
�ʴ�Ϊ���ڶ����ڵ�IVA�壻
��3������Ԫ�ص�ԭ��M����һ��δ�ɶ�p���ӵ�����Ԫ�أ�
�ʴ�Ϊ��Cl��
��4����Ϊ�����ᣬ���ĵ���ʽΪ![]() ����������SO2ˮ��Һ�ɷ���������ԭ��Ӧ����������ǿ�ᣬ��Ӧ����ʽΪHClO+H2O+SO2=H2SO4+HCl��
����������SO2ˮ��Һ�ɷ���������ԭ��Ӧ����������ǿ�ᣬ��Ӧ����ʽΪHClO+H2O+SO2=H2SO4+HCl��
�ʴ�Ϊ��![]() ��HClO+H2O+SO2=H2SO4+HCl��
��HClO+H2O+SO2=H2SO4+HCl��
�����͡��ƶ���
��������
19
����Ŀ�����в���ǰ36��Ԫ�ص����ʻ�ԭ�ӽṹ���±�
| Ԫ�ر�� | Ԫ�����ʻ�ԭ�ӽṹ |
R | ��̬ԭ�ӵ��������3��δ�ɶԵ��ӣ��������2������ |
S | ��������ˮ���ҷ�Ӧ��������Һ�������� |
T | ��̬ԭ��3d�������1������ |
X | �� |
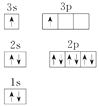
��1��RԪ�صĵ�һ������Ҫ������ͬ�������ڵ�Ԫ�أ�ԭ����________________________________________________________��
��2��SԪ�صĻ��ϼ��Ƿ������ۣ�__________��ԭ����__________________________________�����������Ų�ʽΪ________________________��
��3��TԪ�ص�ԭ��N�ܲ��ϵ�����Ϊ__________����ԭ�ӽṹʾ��ͼΪ__________��
��4��X�ĺ�������Ų�ͼΥ����__________����X���ʡ�������μ����������εȿ����������ȼ��ʱ�����������ɫ�Ĺ⣬����ԭ�ӽṹ��֪ʶ���ͷ����ԭ��____________________________________________________________________��